Document Type : Review Article
Authors
Department of Chemical Engineering, Faculty of Engineering, University of Mohaghegh Ardabili, Ardabil, Iran
Abstract
Carbon nanotubes (CNTs) have gained massive attention given their special and unique features including high surface area, suitable chemical and electrochemical stability, unique mechanical and electronic features, as well as unidimensional structure. Two groups of CNT-based hybrids that have received much attention include nanotube/metal nanoparticles and nanotube/oxide nanoparticles hybrids. There are two major methods for preparing such hybrids: (I) in situ formation of nanoparticles in the presence of nanotubes, (II) use of prefabricated nanoparticles and binding them to the nanotube surface. In the CNTs decoration, the aim is to deposit nanoparticles with adhesivity on the nanotubes surface and a suitable bonding should be established between these two materials. Meanwhile, to achieve a hybrid with improved properties, the initial nanotube features should be kept as much as possible. Three principal methods have been evaluated for CNTs decoration: (I) electrochemical deposition and electroless, (II) chemical deposition, and (III) physical deposition. In this study, the aim is to examine different methods of CNTs decoration and to present the mechanism of methods. For this purpose, different examples related to each method and the effective parameters have been evaluated and discussed. Likewise, recent advances in CNTs decoration with metal and metal oxide nanoparticles have been explored in different applications.
Graphical Abstract
Keywords
Main Subjects
Introduction
The unique electronic and mechanical properties of carbon nanotubes were presented by Iijima in 1991. Accordingly, an extensive range of areas was created regrading absorption, catalysis, sensing, fuel cells and batteries [1-5] for use of this type of nanomaterial. The typical methods for preparing carbon nanotubes include chemical evaporation deposition, electric arc discharge, electrochemical, and laser ablation methods. The main limitations in direct use of synthesized nanotubes include: 1) impurities (amorphous materials, catalyst residuals), 2) structural defects which are structurally different from graphite planes and affect the intrinsic properties of the nanotube, and 3) problem in dissolution of nanotubes in different aquatic and organic solvents, due to high-density Van der Waals interaction between the nanotube [6].
Before functionalizing nanotubes, they should be purified. Three major methods are used for this purpose: (I) oxidation, (II) use of potent acids (non-oxide medium), and (III) annealing at high temperatures and in neutral media [7]. Based on the above points, it can be claimed that functionalization of nanotubes with various chemical compounds could establish a high potential for preparing carbon nanotube-based nanohybrids.
Figure 1(a) displays the increasing trend in the number of publications regarding decoration of carbon nanotubes with metal or corresponding oxides based on Scopus database.
Figure 1(b) reveals that the decorated CNTs with metal or metal oxide nanoparticles are utilized in various research areas including material science, chemistry, engineering as the top three areas.
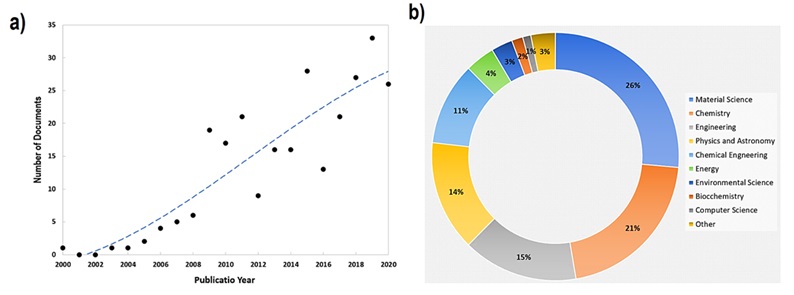
Figure 1. (a) The number of publications regarding metal or metal oxide decorated CNTs based on the Scopus database and (b) several research areas regarding decorated CNTs with metal and metal oxide nanoparticles based on the Scopus database between 2000 and 2020.
To achieve suitable interactions and binding of nanoparticles with the nanotube surface, proper functional groups, and binders should be used. This is because the graphite structure of the nanotube, if being complete with no defect or impurity, is intrinsically inactive, and cannot be used as a suitable substrate for preparing hybrids. Thus, the basis for preparing such hybrids is functionalization of the nanotube surface, such that it would cause the minimum adverse impact on the nanotube intrinsic properties [8].
Generally, there are two major methods for functionalizing nanotubes. The first is covalency method, where chemical groups, through covalent bonds with the nanotube structure, bind to it. The second method involves absorption of macromolecules through noncovalent interactions or coating molecules with various functional groups on the nanotube surface [9].
In the covalence method, the functional groups can be bound to the lateral surface and end of nanotubes. The most important method for functionalizing the end of nanotubes is oxidation, which leads to opening of the nanotube ends. Various functional groups containing oxygen groups such as carboxylic acids, hydroxyls, and aldehydes can be bound to the surface and end of nanotubes through this method. Note that these functional groups can serve as binder for binding other groups or compounds on the nanotube surface. Although the type of structural bond in nanotubes is similar to that of graphite, nanotubes have a higher chemical activity for surface modification than graphite because of their curvature [10].
Another factor in reactivity of nanotubes is presence of structural defects. It is stated that overall, 2% of carbon atoms in single-wall nanotubes lie in non-hexagonal rings, which are somehow considered structural defect. Nevertheless, the covalence bond of compounds leads to degradation of Π -electron transfer within the nanotube structure. This has a great influence on its electronic and mechanical properties [11]. The various methods of covalent functionalization of nanotubes include oxidation reactions of structural defect sites, esterification reaction also known as amidation of oxidized nanotubes, use of ionic fluids, halogenation, increasing radicals, increasing nucleophiles, electrophiles, electrochemical modification, use of plasma, or mechanical-chemical methods [12].
Meanwhile, another method for opening and dispersing bundles of nanotubes is non-covalence functionalization [13]. In this state, the surface of nanotubes is functionalized through Van der Waals or Π- Π interactions. For this purpose, the absorption or coating of multinuclear aromatic compounds, surfactants, polymers, or biomolecules is done. The most important advantage of this method is that the structure and in turn the electrotechnical properties of nanotubes remain constant [14,15].
Here we present a comprehensive review of various methods for carbon nanotube decoration with metal and metal oxide nanoparticles. Accordingly, we studied the relevant literature in four primary categories including electrochemical, electroless, chemical, and atomic layer deposition methods. We carried out a comprehensive classification and discussion on various chemical deposition methods including covalent and non-covalent approaches such as polyol, self-assembly of surfactants, surface sol-gel, modification using dendrimers, binding through Π-Π, hydrophobic, and electrostatic interactions. Furthermore, we represent recent advances in various applications in metal/metal oxide-decorated CNTs.
The main aim of this study is to present a comprehensive overview of various techniques for CNT decoration. We especially, focused on chemical techniques to deposit metal and metal oxide nanoparticles on the CNT surface. More specially, we discussed applying the atomic layer deposition method as a unique self-limiting gas phase technique for CNT decoration. Furthermore, recent advances in using decorated CNTs with metal and metal oxides in various applications such as sensing, pollutant removal, and surface coating are discussed.
Methods of CNTs Decoration with Metal and Metal Oxide Nanoparticles
The term decoration of nanotubes by nanoparticles has been stated in many studies as synthesis of nanotube/nanoparticles hybrids. The first report for decoration of carbon nanotubes with metal nanoparticles goes back to 1994. In that study, ruthenium nanoparticles were evaluated on carbon nanotube substrate as a heterogeneous catalyst. After presentation of this study, a rapid growth has occurred in the fabrication and evaluation of hybrids that are based on carbon nanotubes [16].
Meanwhile, metal nanoparticles have been studied extensively because of their special electronic, optical, and magnetic properties as well as catalytic features [17]. In this regard, noble metals have been evaluated more than others because of their wide applications in chemistry, materials science, and physics [18]. In addition, oxides of minerals and semiconductor materials as well as magnetic compounds constitute another group of nanomaterials whose hybrid preparation has been examined [19].
In many instances, it has been observed that the synthesis and application of nanotube/metal or oxide nanoparticles hybrids, in addition to offering the properties of both materials can cause incidence and emergence of some improved properties as well [20,21]. For example, the hybrids of nanotubes with photocatalytic materials such as titania or zirconia would lead to considerable increase in the photocatalytic activity of the final hybrid [22, 23].
Figure 2 presents different types of methods of synthesizing this type of hybrids as well as their applications.
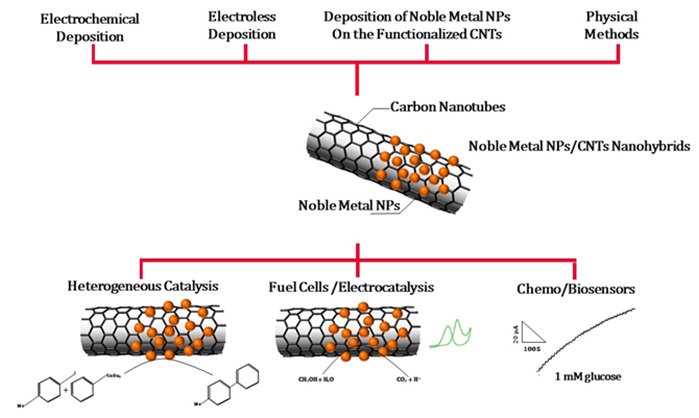
Figure 2. Various methods for the synthesis of CNTs/nanoparticle and related applications [24].
To prepare nanotube/nanoparticles hybrids, there are two general methods: (I) concurrent and in situ formation of nanoparticles in the presence of carbon nanotubes, (II) use of prefabricated nanoparticles and their binding to the surface of nanotube through chemical linkers. The interaction between nanoparticles or their precursors with their carbon nanotube can be of covalence type or weaker bonds including Van der Waals, electrostatic, hydrophobic/hydrogen, and Π- Π interactions [16]. A review on previous studies in this regard suggests that the nanotubes decoration can be performed in liquid, gaseous, and supercritical media.
There are three general methods in the liquid phase including electrochemical deposition, electroless deposition, and chemical deposition. One of the most important practical methods in the gaseous phase is atomic layer deposition (ALD). Studies performed on supercritical media are limited [20, 21, 25-26].
From another aspect, the methods of decorating nanotubes can be categorized into two groups: chemical and physical methods. Since in physical methods, the surface that is subjected to deposition and binding of nanoparticles is the only exposed surface, one cannot completely ensure the complete coating of the entire surface of nanotubes. Although in different studies, suitable control of the size and shape of nanoparticles in the physical method has been mentioned, it is not appropriate for mass production because of its special equipment and high cost [24]. Based on the above-mentioned points, chemical methods in liquid and gas phases will be further investigated while also presenting some examples.
Electrochemical Deposition Method
Electrochemical techniques are an effective method for depositing nanoparticles especially metals on the surface of nanotubes. The basis of this method is indeed the oxidation-reduction reaction in the electrochemical cell. In this state, the carbon nanotube serves as the working electrode in the electrolyte solution containing the metal precursor, and by applying potential or establishing current, electron transfer reaction occurs on the surface of the electrode, which is indeed the nanotube. Complex precursors of metals are often used. As with electrochemical cells, two types of cells can be used: cell with two electrodes (working and auxiliary) or with three electrodes (working, auxiliary, and reference). Typically, the three-electrode type with the reference electrode of Ag/AgCl or standard hydrogen electrode has been used [27, 28].
In this method, the driving force for the reaction on the surface is supplied by establishing potential and external current. The deposition of metal nanoparticles on the working electrode changes its extent of conductivity, and by measuring the current in terms of voltage, the cell performance is evaluated. Through this method, decoration can be done for both the lateral surfaces and end of nanotubes. Carbon nanotubes have dual roles, one as electrode and mold for deposition of nanoparticles and the other as a conductive cord which links nanoparticles to each other [24, 29].
Thus, first an electrode of carbon nanotubes should be prepared. For this purpose, the carbon nanotube can be synthesized on an insulated substrate such as silica via CVD method, and then the nanotubes can be linked to each other using a conductive wire such as titanium or gold, so that it could be used in electrochemical cells. In this method, lithography techniques are employed for incorporating the catalyst and binding titanium wire to the nanotubes.
Figure 3 presents a sample of this type of electrode [30]. According to Figure 3, for evaluating and conducting the experiment, a part of the electrolyte solution containing the precursor is poured onto the electrode, and by establishing the current, reduction reaction of the metal ion occurs on the nanotube surface. In addition, for preventing contact between the conductive wire and the electrolyte solution, an inert coating is created on it in the electrolyte medium. In this method, the concentration of the metal salt, the deposition time, and the potential applied for nucleation are among the control parameters for the extent of coating and size of particles [31].
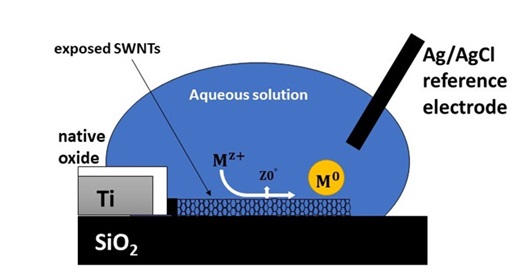
Figure 3. Schematic of two-electrode cell used in Electrochemical Deposition method [30].
In another method, instead of creating a new electrode, a coating of the nanotube suspension along with metal precursor is created on typical electrodes such as glassy carbon (GC) as cast. It is then air dried and used as the working electrode in the cell [32].
In another study, two types of two- and three-electrode cells were prepared on silica insulator material, whose performance was evaluated for decoration of platinum and silver. A schema of these two types of cells is shown in Figure 4. In type a, there are two electrodes. In this state, the carbon nanotube along with part of the gold functions as the working electrode, while in state b, with three electrodes, the protective coating has been created on gold [33].
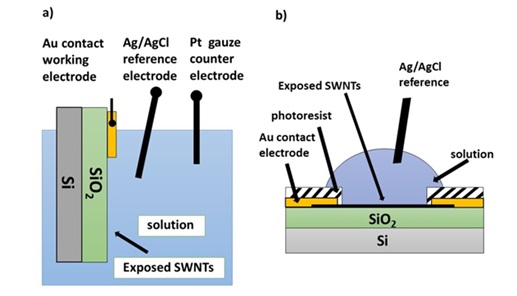
Figure 4. Two different types of two- and three-electrode cells prepared for decoration of platinum and silver on carbon nanotubes [33].
A major problem in this method is controlling the size and distribution of particles on the surface. To improve the dispersion of nanoparticles on the surface and reduce their size in this method, various corrective techniques have been employed. One example is use of electrolyte solution as a stabilizer of nanoparticles on the nanotube surface and prevention from their aggregation. Another method is creating functional groups on the nanotube surface before the deposition operations.
Oxide functional groups [24] or aminobenzenes have been used in this application, which have created 2-3 nm nanoparticles [29].
Electroless Deposition Method
The electroless deposition method, as with the electrochemical method, is suitable for surface coating. Typically, in this method, only one electrode along with the electrolyte solution is used. For performing the reaction on the surface, a reducing compound or catalyst should be used in the solution. There is no need for external potential for occurrence of reaction on the surface [34-36]. The basis of the electroless method for decoration of nanotubes is similar to occurrence of a galvanic displacement reaction. The necessary driving force for the reaction in a galvanic cell is oxidation/reduction potential difference of two metal compounds. Accordingly, one becomes oxidized (anode) and the other becomes reduced (cathode). Typically, reducing compounds are used in the solution such as hypophosphite and dimethyl amine borane [37, 38]. The first report related to decoration of single wall nanotubes via this method was presented by Dai et al. in 2002. This group witnessed spontaneous reduction of the precursor of gold and platinum complex in the presence of carbon nanotube without using any reducing agent. According to the report of this group, considering the lower oxidation/reduction potential of the nanotube (0.5 V) compared to gold (1 V) and platinum (0.775 V), these precursors can be reduced in the presence of nanotube. The limitation of this method is that it is only suitable for reduction and deposition of metals that have oxidation/reduction potential higher than that of the nanotube [37].
As displayed in Figure 5, to resolve the limitation of this method, surface enhanced electroless deposition (SEED) technique has been used. In this state, a metal substrate with a lower oxidation/reduction potential compared to the ion of interest in the electrolyte solution can be used to create the driving force required for its reduction on the carbon nanotube [39].
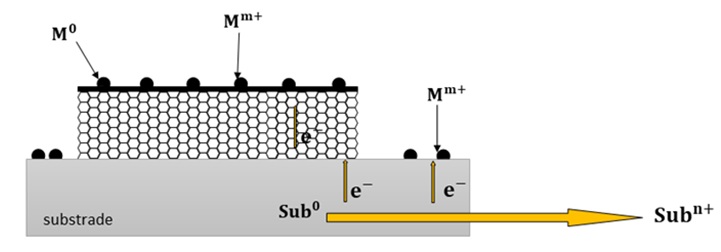
Figure 5. Schematic of the electrode in SEED method [39].
Using a copper substrate, metal ions such as gold, platinum, and palladium can be reduced on the surface of nanotube. It has also been used for creating copper nanoparticles (0.34 V) and silver nanoparticles (0.37 V) on the nanotube surface from zinc metal substrate (0.67 V) [39]. According to Figure 6, reduced carbon nanotubes can be used for the electroless deposition. Specifically, first the nanotubes are reduced by sodium metal, and then the deposition of gold and platinum nanoparticles occurs under electroless conditions without any need to surfactants or reducing agents or metal substrates, only in one stage [40].
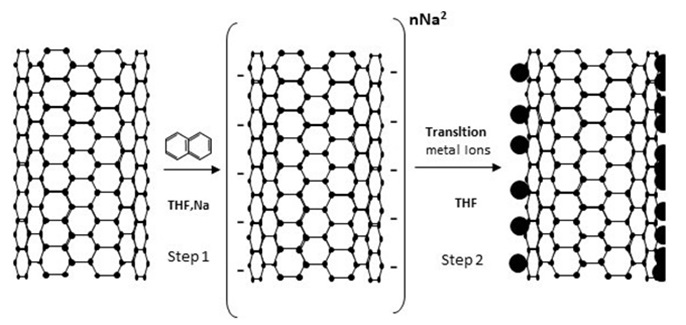
Figure 6. Schematic of metal ion decoration on reduced CNTs by sodium [40].
Chemical Deposition
In this method, essentially the reduction or hydrolysis of metal precursors in the presence of carbon nanotubes leads to formation of nanoparticles on its surface. The deposition on the surface is often as Van der Waals interactions, while also being sufficiently strong. Under these conditions, chemical compounds are used for hydrolysis and reduction, or heat and light are employed for reducing the metal precursors. Furthermore, to improve and enhance the reaction kinetics, external forces such as ultrasound and microwave have also been utilized [41-43]. The important point in this type of method is the competition between homogeneous and heterogeneous nucleation in the liquid medium. This is because considering the presence of precursors as well as reduction and hydrolysis agents in the solution, the reaction occurs by default inside liquid and homogeneously. Meanwhile, concerning the neutral nature of carbon nanotubes, it is expected that they can only function as a substrate for deposition of particles. Nevertheless, comparison of carbon nanotubes with graphite or amorphous carbon under equal reaction conditions suggests the effective role of nanotubes in controlling the size and distribution of the formed nanoparticles [44]. In other words, carbon nanotubes can function as a mold for adjusting and controlling the size of particles. However, this feature will be observed only when the nanotubes surface has active functional groups. These functional groups can be indeed the structural defects of nanotubes or other compounds that have been bound to the nanotube surface or its end through covalent or noncovalent bonds secondarily and along some operations [45-46]. Different types of compounds have been used as binder of nanoparticles and nanotubes. The examples include surfactants, ion polymers, ionic liquids, dendrimers, and derivatives of circular aromatic compounds [47-51]. Furthermore, in situ synthesis of nanoparticles can be done in different methods, including:
)1( In situ reduction in the liquid medium using surfactants (polyol method),
)2( Hydrothermal/solvothermal method, and
)3( Sol-gel method.
Meanwhile, different types of noncovalent interactions can be used for stabilizing the binder compound on the nanotube surface. The examples include Π- Π, electrostatic, and hydrophilic interactions along with hydrogen bond. Each of the mentioned instances will be further explained by providing some examples [52, 53].
Covalent bond
The nanotubes surface can be functionalized using various functional groups such as chlorides, thiols, or carboxylates in a covalent way, after which this agent can be employed as superficial active sites in the decoration of metal and oxide particles. One of the compounds that is used for this purpose is ionic liquids [54, 55].
For example, ionic liquid based on imidazole salt has been used for preparing nanotube/platinum hybrid. This type of ionic liquid binds from the head of its amine groups to the carboxylic groups of the surface, thereby forming amide. Ionic liquids facilitate electron transfer between the metal ion and nanotube and improve it. The replacement potential of ionic liquid anions with the negative ion of platinum leads to suitable stability of the precursor around the nanotube. It has also been stated that due to the low surface tension of ionic liquids, we observe tiny particles, since Ostwald ripening phenomenon occurs weakly. As observed in Figure 7, after electrostatic absorption of the metal precursor on the surface using a reducing agent such as ascorbic acid, platinum has been reduced as metal on the surface and gets deposited [55].
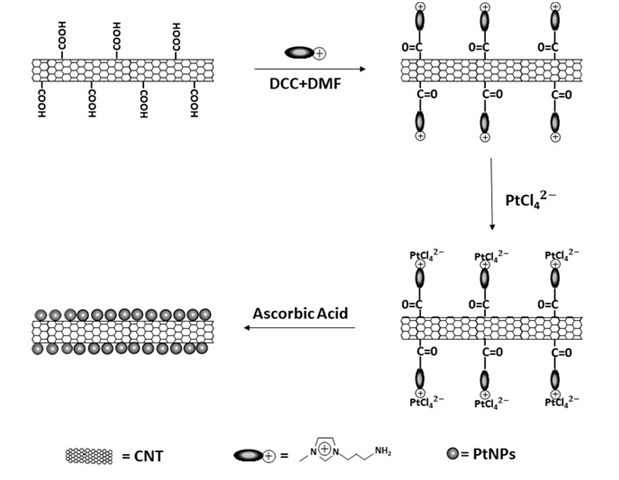
Figure 7. A schema of the synthesis of CNT/ionic liquid/Pt hybrid [55].
Polyol method
This method is essentially a technique for preparing suspension from metal nanoparticles; the reaction can be done in the presence of nanotubes by adding carbon nanotubes to that as the substrate [56]. This method is based on the reflux of metal salt solution in a polyol solvent at 393-443 K. A general schema of this method has been presented in Figure 8. Typically, ethylene glycol is employed for this purpose. Homogeneous degradation of polyol results in release of the metal ion reducing compound. This method has been utilized for preparing nanotube hybrids with metals as well as metal alloys and oxides. Furthermore, the heating stage of this method has also been done under microwave or ultrasound conditions [29].
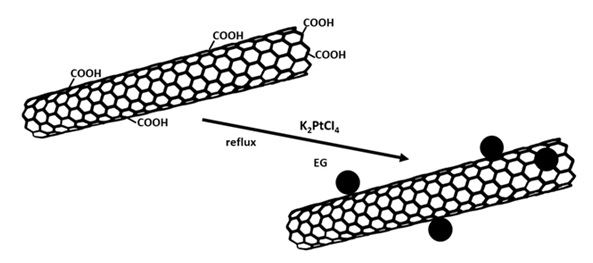
Figure 8. A general schema of preparation of the carbon nanotube/metal hybrid via polyol method [29].
Figure 9 depicts the decoration of carbon nanotube with erbium nanoparticles. First, the nanotube surface is oxidized in a strong acidic medium, and a series of oxygenated functional groups is created on its surface [57].
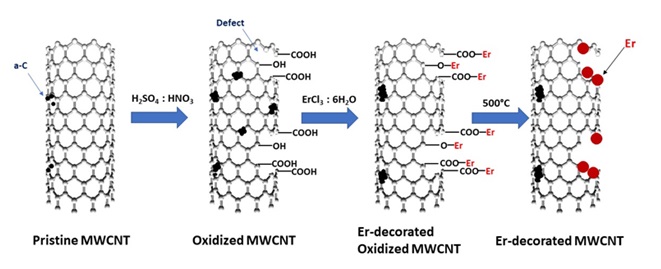
Figure 9. Decoration of carbon nanotubes with erbium nanoparticles [57].
These operations also cause development of structural defects and amorphous carbon. Next, in the medium containing ethylene glycol and erbium carbide at 150 °C under reflux and for 4 h, they are placed on the surface as metal particles and after washing and drying under vacuum, the desired hybrid is achieved [57]. In another study, this method has been employed for preparing carbon nanotube/iron oxide hybrid. A schema of its preparation method has been presented in Figure 10. This time, for absorption of iron cations on the nanotube surface, first a layer of CTAB surfactant has been used after which coating has been done with the poly sodium styrene sulfonate ionic polymer [58].
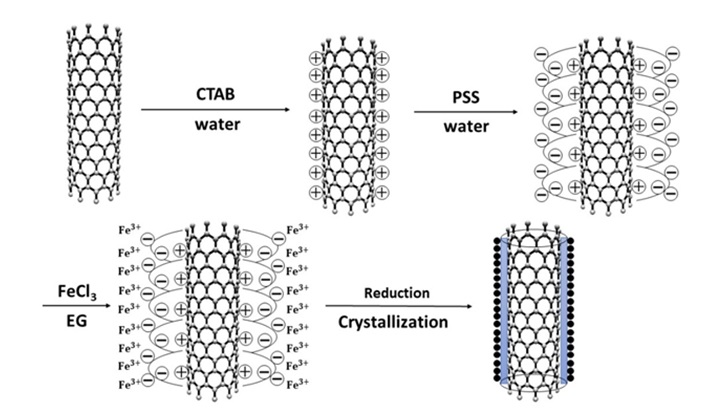
Figure 10. Preparation of the carbon nanotube/iron oxide hybrid via polyol method [58].
According to Figure 10, existence of negative charge high density on the surface has led to in situ reduction of part of the iron ions, and in some ways, it has become stabilized on the surface because of the very high electrostatic interaction, and then it has been reduced as polyol and deposited on the nanotube surface [58]. In this method, the difference of the reduction power of polyol solvent that is used can affect the size and distribution of particles on the nanotube. Investigations have also shown that in case of using carbon nanotube without functionalizing its surface, the created metal particles will be very large, indicating their nucleation and homogeneous growth and the ineffectiveness of the carbon nanotube in that form [59].
Self-assembly of surfactants on the surface of carbon nanotubes
Another method of binding nanoparticles to the surface of carbon nanotubes is applying structures resulting from self-decoration of surfactants on the carbon nanotube surface [60].
Research has shown that amphiphilic surfactants at high concentrations above the critical concentration of micelle formation and in the presence of carbon nanotubes take a pseudo-micelle form, preventing aggregation of the abundance of nanotubes and improving their water-solubility [61, 62]. Surfactants with a long alkyl chain as well as active and polar head have been employed for this purpose. Meanwhile, sodium dodecyl sulfate is one of the most practical ones in this regard. This type of surfactants and structures resulting from them are known as soft template, since such a structure only forms in the presence of carbon nanotube [60, 63].
Figure 11 indicates the way the nanotube/palladium hybrid is prepared using this type of mold. After formation of pseudo-micelles, there is strong electrostatic interaction between the sulfate groups and metal ion leads to its high stability on the surface, after which it is changed into palladium metal using an optical reducing agent [62].
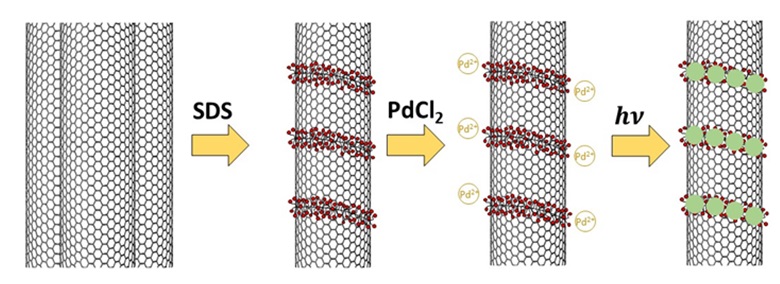
Figure 11. The method of synthesis of carbon nanotube/palladium hybrid via surfactant as templates [62].
Surface sol-gel method
Sol-gel method has also been used for decoration of carbon nanotubes with metal nanoparticles and preparation of hybrids [64, 65]. In a study, the combination of sol-gel method with ultrasound was used, whereby the researchers could prepare different types of hybrids based on carbon nanotubes with metals such as gold, platinum, palladium, and bimetals [66, 67]. Figure 12 presents the method of preparation of this type of hybrid.
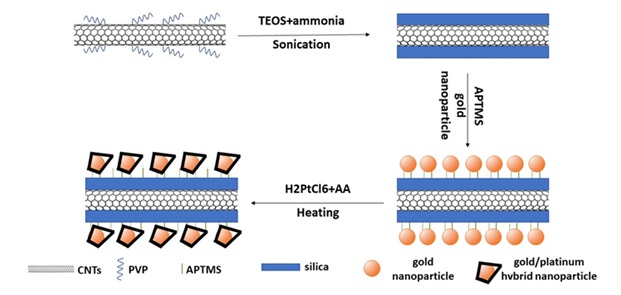
Figure 12. The method of preparation of platinum/gold nanoparticles on the coaxial silica/nanotube nano wire via the sol-gel method [64].
According to Figure 12, in this method first a coating of PVP polymer is created on the nanotube surface. Next, via sol-gel method, a silica coating has been created on the carbon nanotube surface using alkoxide, ammonium precursor through ultrasound. At this stage, a coaxial nano wire of nanotube and silica has been prepared. Thereafter, it has functionalized the surface using APTMS which is a silicate compound with methyl and amine groups. The already prepared both nanoparticles are then immobilized on the surface using amine groups, after which reduction of the platinum metal precursor occurs in the presence of ascorbic acid and heat, whereby platinum nanoparticles are formed on the gold surface [64].
Modification of nanotubes using dendrimers
Another group of compounds that can create good binding with the nanotube surface and nanoparticles are dendrimers. These compounds bind the nanotube through covalent bond, and through the numerous functional groups that exist in them they can be employed for preparing hybrids of metals (gold, copper, and silver) as well as oxides (titania and silica) with carbon nanotube [68, 69].
Figure 13 presents the method of preparation of metal and oxide hybrids using dendrimers. Based on Figure 13, fourth-generation poly amidoamine with the NH2 terminal groups has been used as the binder of nanoparticles and carbon nanotube [70].
For this purpose, first the nanotube surface has been functionalized using chlorinated groups, and then the dendrimer is bound to the nanotube through formation of amide bonds. Next, dispersion of the modified nanotube occurs with dendrimer inside water through vigorous mixing, to which the desired precursors of nanoparticles are added [71]. For preparing oxides, alkoxide precursors have been used, whereby over time hydrolysis and formation of oxide nanoparticles occur. On the other hand, for the hybrid of metals, reducing agents are used. It has been found that the rate of hydrolysis of precursors determines the morphology of the final oxide particles.
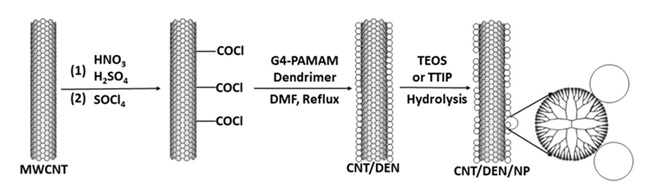
Figure 13. The synthesis of CNT/metal or CNT/metal oxide using dendrimers [71].
Also, two mechanisms have been proposed for interaction between nanoparticles and dendrimer. The initial stage is encapsulation of nanoparticles inside the dendrimer which has been observed typically at particle size smaller than the dendrimer size, while the second stage is coordination of nanoparticles on dendrimers because of their numerous functional groups. This state occurs when the size of particles is larger than that of dendrimers. A controlled experiment has been done for determining the effect of dendrimers, without their presence, and with constancy of other conditions [72].
Noncovalent binding methods
Binding through Π-Π interactions
Another group of compounds that has attracted much attention for binding nanoparticles the carbon nanotubes are two- or multifunctional compounds, such that from one head they would have aryl or polyaromatic groups, while from the other head they would have polar functional groups such as thiol, carboxyl, or amine. These compounds typically establish a very good interaction with the six rings of the nanotube wall from the nonpolar head, and bind to it [73, 74].
Using these compounds, methanol or oxide nanoparticles can be deposited on the nanoparticles’ surface by choosing suitable binders. This method has attracted a great deal of attention because of its simplicity and more importantly maintaining the structure of the carbon nanotube electromechanically as well as the possibility of establishing suitable size of nanoparticles on the surface [75-79]. For example, triphenyl phosphine has been used for preparing platinum-carbon nanotube hybrid [76], aminopyrine has been employed in preparing platinum/ruthenium bimetallic compound [78], and pyrene aldehyde derivatives have been utilized for preparing magnetic nanoparticles such as iron oxide, cobalt, cobalt/platinum [75], and 1-pyrine butyric acid N-hydroxy succinide ester (PANHS) has been applied for preparing gold nanoparticles [77].
Also, phenyl phosphonic acid has been used for preparing the hybrid of different types of oxides on nanotubes [79]. In this method, again in situ formation of nanoparticles is possible, and prefabricated nanoparticles can also be used.
Binding via hydrophobic interactions
Another type of interaction between nanoparticles and carbon nanotubes using binders can be in the form of hydrophobic forces between the alkyl chain and the nanotube surface. Likewise, the functional groups of the binder can establish a hydrogen bond with the carboxylic groups of the nanotube surface. Figure 14 indicates a schema of the hybrid system that has been formed based on this type of interaction [80].
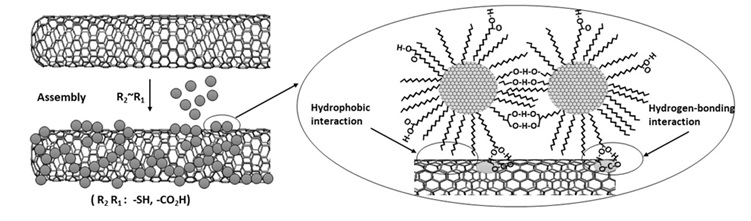
Figure 14. The binding of nanoparticles on CNT surface through hydrophobic interactions and hydrogen bindings [80].
Binding through electrostatic forces
To establish such interactions, various types of ionic poly electrolytes have been used such as polyethylene imine (PEI), Poly(diallyldimethylammonium chloride) (PDAC), which are cationic polymers, as well as poly sodium styrene sulfonate anionic polymer. These compounds create a polymeric charged layer or layer by layer on the surface, thereby causing high absorption of precursors or nanoparticles through electrostatic forces [81].
Figure 15 presents a schema of this method for preparation of gold hybrid by creating a PEI polymer layer. The results of this study indicated that PEI has a very strong physical absorption on the surface, and thus creates a suitable layer on the surface for absorption of gold anions. This type of polymer in addition to a stabilizing role for nanoparticles on the surface serves as a reducing agent of gold precursor [82].
Studies have indicated that the extent of polymer affects the final particle size of the hybrid; at low concentrations due to decreased stabilizing power, we will observe enlarged gold particle size on the surface. Meanwhile, the operational conditions of the reaction such as temperature and time directly influence the extent of superficial coating of gold nanoparticles on the surface [82].
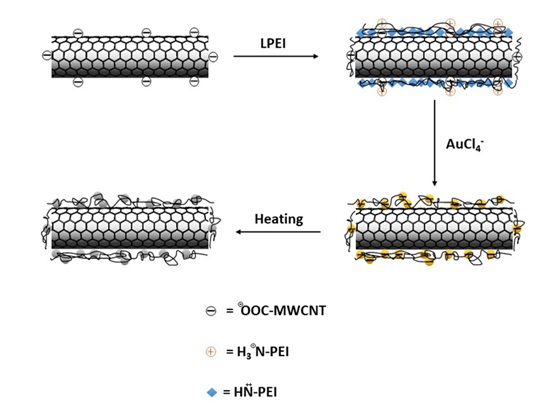
Figure 15. The interaction of PEI cationic polymer with the oxidized nanotube through electrostatic forces and strong physical absorption [82].
As observed in Figure 16, with repetition of polymer layers and changes in the superficial charge, various types of anionic and cationic precursors can be absorbed on the surface, and through their reduction or hydrolysis, hybrid of nanoparticles on carbon nanotube can be achieved [83].
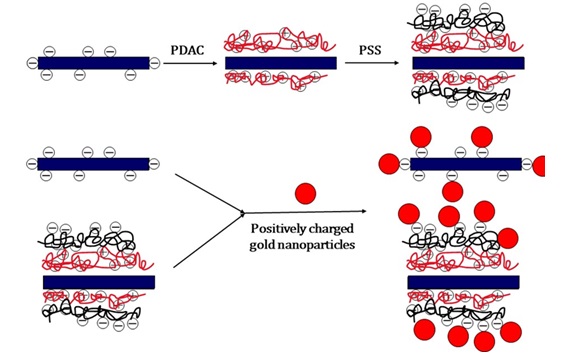
Figure 16. The layer-by-layer method for creating a surface with different charges and immobilization of nanoparticles [83].
Atomic Layer Deposition Method
Atomic layer deposition (ALD) method is often used for correcting the surface of single wall nanotubes and covering their structural defects for enhancing their stability and lifetime. Since complete coating of the nanotube surface is not suitable in such applications and concerning the nature of the ALD method, the decoration of carbon nanotubes via this method acts fully selectively and will occur only on the active sites of the nanotube surface, which are indeed its defects. Meanwhile, the self-limiting nature of this method provides a very suitable control on the size and morphology of particles [84]. To confirm the selective operation of this method in the surface coating, in a study the number of deposition cycles was increased from 37 to 200. In that study, the aim was to cover the superficial defects of the single wall nanotube with zinc oxide nanoparticles. As the number of cycles increased, no film was observed on the surface. Thus, this research group suggested that only structural defects and the impurities present in carbon nanotube function as a strong site of chemical absorption for zinc metal precursor (diethylzinc) and water, and will lead to formation of nanoparticles in these regions of the surface [85].
Different types of nitrogen across the nanotube surface and the occurrence of reaction and absorption in the first cycle of ALD are demonstrated in Figure 17. At high temperatures, the extent of activity of the graphite type decreases for chemical absorption of the tin chloride precursor, and it is the pyridine type that mostly absorbs. Given the density of the active sites at high temperatures regarding initial absorption the tin chloride will have reduction in the very first layer. Meanwhile, in the subsequent layers, temperature elevation again leads to reduction of the superficial hydroxyl groups [86].
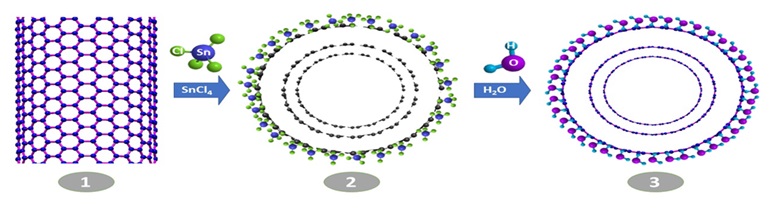
Figure 17. Different types of nitrogen across the nanotube surface and occurrence of reaction as well as a reduction in the first ALD cycle [86].
The way temperature affects the mechanism of ALD reaction as well as the size and morphology of the created final phase are displayed in Figure 18. Another affected parameter by temperature is the mechanism of the reaction of chlorine precursor with the surface. Water absorption is absolutely physical and with temperature alteration, the same mechanism of ligand displacement will be governed in it. However, regarding chemical absorption of tin chloride, it is not the case and the mechanism of ligand displacement and that of chlorination occur at low and high temperatures, respectively. Thus, at high temperatures, in each cycle, we witness formation of several layers of tin oxide on top of each other, while at low temperatures, a fully single layer develops in each cycle. That is why the distribution of particle size at high temperatures is observed in comparison to lower temperatures. This study well indicates the ability of ALD method in selectivity and control of the size, phase, and distribution of particles on the surface of carbon nanotube [86].
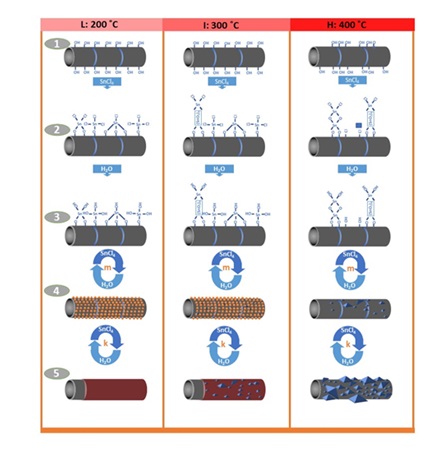
Figure 18. The effect of temperature on ALD mechanism and its impacts on the size and morphology of final structure [86].
Recent Advances in CNTs Decoration with Metal and Metal Oxide Nanoparticles
Carbon nanotubes with decoration of metal nanoparticles such as silver, titanium, copper, iron, nickel, iridium, zinc, platinum, and palladium, as well as their metal oxides have been synthesized and studied in different applications [87-92]. The metal and/or metal oxide decorated CNTs have been studies in various applications such as sensing, pollutants removal, and surface coating [93-98]. Chemical evaporation method has been employed for decoration of prefabricated metal and metal oxide nanoparticles on CNT. For this purpose, the desired values of nanomaterials and nanotube are mixed in the presence of dimethyl formamide under ultrasound radiation, and eventually through solvent evaporation at room temperature, the final structure can be achieved [87].
Wet chemical method has also been used for decoration of titanium oxide nanoparticles on CNT with different percentages of metal oxide. According to that study, at higher concentrations of titanium tetrachloride as tin precursor, a more thorough coverage of the external surface of CNT was achieved. Also, the study of the effect of synthesis time on the extent of decoration and coating of the nanotube surface indicated that over time, in addition to coat the nanotubes surface, nanoparticles also penetrated into the nanotube [88].
In a study, decoration of silver nanoparticles was performed via electron beam evaporation on carbon nanotubes. This structure has been employed for detection of acetone gas at ambient temperature as well as 50-800 ppm concentrations. That study found that the sensing response in the Ag-CNT structure was around 2-3 times as large as in CNT structure alone. Furthermore, the structure resulting from decoration of silver nanoparticles on CNT enjoyed greater selectivity, stability, and sensitivity compared to CNT alone [90]. Thermal evaporation method of zinc metal powder in the presence of CNT has been used for decoration of zinc oxide on nanotubes and employed as a gas sensor in the application of nitrogen dioxide detection with 1-10 ppm concentration. Zinc oxide nanoparticles led to improvements in the response and shortening of the sensing response time of ZnO-CNT [99]. A gas sensor created from decoration of iridium oxide on CNT has been utilized for detection of ammonium and nitrogen dioxide gases at 25, 100, and 150 °C. It was observed that addition of nanoparticles to the carbon nanotube resulted in considerable increase in the sensing response to both gases. Likewise, the selectivity of the created sensor to different gases suggested high response to nitrogen dioxide in comparison to other gases including ammonium, carbon monoxide, hydrogen, and hexane [100].
Decoration of phthalocyanine and metal oxide nanoparticles including zinc oxide and iron oxide on carbon nanotube has been employed for modifying carbon electrode. This electrode, as electrocatalyst for dopamine detection, has shown sensitivity of 1.45 µA/µM and detection limit of 0.75 µM [101]. For detection of methoxy phenol, decoration of chromium oxide nanoparticles on carbon nanotube has been utilized as a carbon electrode surface coating. The sensor created using this method showed linear behavior within the 0.01-0.1 nM range. The sensitivity and detection limit of the modified electrode were reported as 1.5 µA/cm2 µM and 0.06 nM, respectively [102].
Thermal evaporation has been used for decoration of metal nanoparticles including palladium, cobalt, and nickel on CNT for fabricating a high-performance electrocatalyst in water splitting reaction. The synthesized electrocatalyst contained slight amounts of the catalyst at around 10-2 mg/cm2 at working voltage of 1.5 V, where the current's output showed stability higher than 2 mA/cm2 within at least 20 h [103]. Also, NiS/Fe3O4 decoration on carbon nanotube has been utilized as an electrocatalyst in water splitting reaction. The current density of this structure at low voltage of 243 mV was reported as around 10 mA/cm2 [104].
Decoration of manganese oxide nanoparticles on carbon-graphene nanotube three-dimensional lattice as anode in lithium-ion battery has been reported for improving the battery performance. The synthesized electrode showed a high electrochemical performance including high specific capacity of around 526.7 mAh/g together with 98% capacity recovery in 1400 cycles [105]. In a study, the decoration of nickel-cobalt oxide nanoparticles with core/shell morphology was used on CNT for improving the supercapacitor performance and enhancing the current density. The created supercapacitor had current density and power of 72.5 Wh/kg and 1.4 kW/kg, respectively [106].
The decoration of silver nanoparticles on CNT was employed as filler in the synthesis of polymer nanocomposite including polypropylene and polystyrene. That study showed the mechanical and electrical conductivity properties of the polymers improved upon the addition of Ag-CNT nanostructure. Also, use of the solution mixing method in comparison to melt mixing method resulted in more suitable dispersion of Ag-CNT across the polymer lattice [107].
It has been shown that the decoration of Fe3O4 nanoparticles on carbon nanotube led to partial increase in the pH of point zero charge (PZC) of the nanotube, and accordingly it can be potentially used in various applications [108].
The study of the process of sulfur hexafluoride absorption on a structure consisting of decoration of platinum and palladium metal nanoparticles concurrently on CNT via DFT method has been reported. In that study, absorption of the species resulting from degradation of sulfur hexafluoride including SOF2 and So2F2 has been investigated, showing that this structure would have greater sensitivity to SOF2 [109]. Another study improved the optical properties of carbon nanotube after decoration of cadmium oxide 22-nm nanoparticles through laser pulse deposition method [110]. Improvement of the formaldehyde oxidation process at low temperatures using decoration of manganese oxide nanoparticles on CNT with 40% constant has also been reported. This catalyst at 30 °C showed 95% removal of formaldehyde with 100 ppm concentration [111]. The decoration of carbon and graphene nanotube with nickel-based metal organic framework via solvothermal single-stage method has also been reported. The absorption performance of this structure has been examined for methylene blue as the absorbate model. The absorption capacity of Ni-MOF-CNT for methylene blue was achieved as about 160-180 mg/g, which in comparison to Ni-MOF alone has grown by around 2.5 times [112]. The synthesis of magnetic decorated CNTs has been reported in several studies [113]. Moreover, their use in organic pollutant removal and use as organometallic nanocatalyst have been studied [114,115]. Serotonin detection under in vitro conditions has also been reported using a structure consisting of copper oxide on carbon nanotube decoration, ultimately being activated by platinum nanoparticles. Detection limit of this electrochemical sensor was found as about 3 nM with high stability and replicability [116].
Conclusion
Decoration of carbon nanotubes with metal nanoparticles and oxides has attracted much attention given their numerous important applications. Overall, the decoration of nanotubes can be done in liquid, gaseous, or supercritical media. In the liquid phase, electrochemical, electroless, and chemical methods as well as different types of methods including sol-gel, hydrothermal, polyol, micro emulsion, and reduction in the presence of surfactants have been employed. In the gaseous phase, atomic layer deposition method is utilized for decoration of nanotubes with oxides.
Electrochemical and electroless methods have attracted industrial attention because of their high speed, suitable phase purity, simple equipment, operation at room temperature, and suitable control over the size and shape of particles. Electroless method, when compared with the electrochemical counterpart, has a lower deposition rate. Nevertheless, in this method there is no need to apply external potential or add reducing materials to the catalyst. In these methods, metal complexes and nitrites are often used and have been examined extensively for decoration of nanotubes with metals.
In chemical methods, although the mechanism and control become more complicated, and the reaction of formation of nanoparticles is difficult, these methods can be employed for a wide range of precursors as well as metal nanoparticles and oxides. In this method, metal alkoxides, complexes, chlorides, alkyls, and nitrates have been utilized.
In the chemical method, the decoration can be done in two ways: (i) in situ reduction of precursors in the presence of nanotubes and (ii) direct use of nanoparticles and binding them to the nanotube surface using binders. It has also been observed that usage of carbon nanotubes without any superficial correction or functionalization cannot provide a suitable size and distribution of nanoparticles on the nanotube surface. In these conditions, nucleation and growth of nanoparticles often occur homogeneously and inside the liquid phase.
On the other hand, functionalization of the surface of nanotubes would effectively lead to improvements in the selected deposition of nanoparticles on the nanotube surface and superficial active sites. Although development of covalent bonds within nanotube surface through binders would create a strong bond, it causes shortening of nanotubes and decline of their electromechanical properties. In contrast, non-covalent methods have attracted much attention because of constancy of the intrinsic properties of the nanotube and their simplicity.
The type of binder used as well as the type of its interaction with the nanotube surface on the one hand and the surface of nanoparticles on the other influence the size and distribution of nanoparticles along with the final properties of the hybrid. As mentioned earlier, different types of binders have been employed including small molecule surfactants and ionic polymers. The major limitation of these compounds is the limited number of sites through which they can attach to the surface (four surfactants) and sensitivity to environmental conditions such as ionic power pH.
Meanwhile, most of the mentioned methods are only suitable for decoration of metals or oxides separately. Thus, a suitable binder should have a three-dimensional structure with different types of functional groups including hydrophilic, hydrophobic, dendrimer, and linear alkyl segments so that in addition to providing suitable and strong binding to the surface, they can also lead to reduction of metal precursors and formation of metal nanoparticles as well as hydrolysis of precursors for reaching oxides.
It has also been shown that atomic layer deposition method in the gaseous phase is an effective method. In this state, homogeneous nucleation is impossible to occur, while it has great potential for controlling the size, distribution, and morphology of final particles.
Disclosure Statement
No potential conflict of interest was reported by the authors.
ORCID
Fahimeh H. Saboor : 0000-0001-7729-0673


