Document Type : Review Article
Authors
1 Department of Materials Engineering, School of Engineering, Yasouj University, Yasouj, Iran
2 Department of Chemical and Materials Engineering, University of Alberta, Edmonton, Alberta, Canada
Abstract
Austenitic stainless steel 316's role in industrial applications has spurred extensive but fragmented studies, presenting challenges in synthesizing its diverse properties. This study comprehensively investigates its fracture properties, analyzing the interplay of mechanical traits, microstructural nuances, strain rates, operational temperatures, hydrogen and helium impacts, heat treatment effects, and fracture behaviors across varying operational parameters. Analysis reveals a robust correlation between microstructure and mechanical characteristics, specially yield stress and fracture topography. Predictive models like Hall-Petch equation and Gibson-Ashby micromechanical model adeptly project these mechanical attributes. Deformation strain-rate surpasses relative porosity density in impact. Higher relative density prompts increased slip bands and grain deformation at constant strain rates, indicating local shear as the primary fracture mode, evident from observed shear bands. Hydrogen's influence, though delayed, assumes a secondary dominant deformation mechanism. While low strain rates do not alter failure modes due to hydrogen damage, its primary impact lies in reducing stress required for dislocation displacement and crack propagation, thereby diminishing tensile strength. External hydrogen exhibits a pronounced effect in some instances. Heat treatment significantly modifies the ferrite-cementite phase interface, impacting fracture morphology, notably at higher temperatures. Controlled annealing enhances fracture resistance at the expense of potential strength reduction, necessitating cautious execution due to heightened hydrogen embrittlement risk from reduced grain boundary chromium. This study seeks to consolidate insights into 316 SS fracture behavior, offering future research directions and practical implications for optimizing its performance in varied industrial settings.
Graphical Abstract
Keywords
Main Subjects
Introduction
The exceptional formability and corrosion resistance of austenitic stainless steels (SS) make them a popular choice for many engineering applications. However, their low yield strength generally limits their use as efficient structural components for energy absorption. Modifying fine-grained microstructures using severe plastic deformation has been particularly important in the development of high-strength steels [1-9]. While strength steadily increases with this technique, ductility and fracture toughness are noticeably reduced. Reducing fracture toughness is crucial for halting dislocation activity in nanostructured grains. The structure reliability and the potential for engineering applications of components containing nanoparticles are constrained by their low defect resistance because numerous large-angle grain boundaries within a three-dimensional network can act as direct nucleation sites and paths for crack propagation [1]. Therefore, studying the fracture properties of SS 316 is of utmost important if such steel is needed to be used in the industry. The present article aims to review such properties and parameters affecting it.
Mechanical Characteristics of the 316 Stainless Steels
In a study [4], the authors discuss investigating the relationship between the microstructure, strength, and fracture topography of 316L stainless steel. The study employed the Hall-Petch equation to analyze how the material's fracture surface dimensions (DF) and microstructure grain size (DM) impact its strength. During the test, the grain size was measured, and its connection to DF -the irregularity of the relevant fracture surface- was established. This connection suggests that as fracture surface roughness decreases (lower DF values), the grain size increases (lower DM values). The steel underwent heat treatment at temperatures of 904, 1010, 1095, and 1194 °C to achieve coaxial grain microstructures. Subsequently, tensile tests were conducted on the samples at room temperature, and a scanning electron microscope was employed to examine the fractured surfaces. DF was calculated using a slitting method, while DM values were sourced from previously published research. The results indicate a correlation between grain size, DM, mechanical characteristics, and DF in 316L steel, which could potentially be extrapolated to many fine-grained metal alloys crucially utilized in engineering applications [4].
The research demonstrates the application of the Hall-Petch equation in establishing a connection between the dimensions of the fractured surface and the grain size concerning the strength of 316L austenitic stainless steel. The findings indicated a notable correlation among refined grains, yield stress, and fracture topography within the studied materials [4].
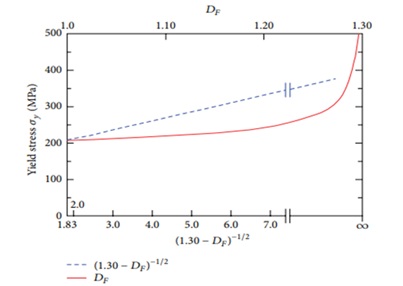
Figure 1. Hall-Petch correlations for AISI 316L austenitic stainless steel between yield stress and fracture surface dimensions [4].
Another study [10], the tensile strength and fracture toughness of 316L stainless steel featuring a cellular microstructure produced via selective laser melting (SLM). This study investigated the impact of various microstructures on density, tensile strength, and fracture toughness related to the cellular formation. The findings are as follows: (1) The solid bases' relative density in both vertical and horizontal directions reached 96.25%, while the cellular microstructure density was recorded at 1.35 g/cm³ for both orientations. (2) Vertically fabricated samples exhibited nearly 60% higher tensile and ultimate yield strengths compared to horizontally produce ones. In addition, vertical samples showcased approximately 40% higher elongation than their horizontal counterparts.
This difference could be attributed to the comparatively weaker nature of horizontally manufactured unit cells. (3) Tensile specimens within the cellular microstructure cracked near the end solid plane. This occurrence stemmed from a sudden density shift, causing a change in toughness and consequent stress concentration at the interface, resulting in fracture. (1) The fracture toughness of 316L stainless steel's cellular microstructure was measured at 3.3 MPa·√m for horizontally generated samples and 4.3 MPa·√m for vertically produced ones. While factors such as base metal density and loading rate may influence fracture toughness, the microstructure's orientation significantly impacts it. (2) The Gibson-Ashby micromechanical model proves beneficial in determining fracture toughness for materials with a cellular microstructure. The findings from the single edge notch bend (SENB) and the micromechanical model exhibit no discernible difference, ranging between 0.2 to 0.5 MPa·√m [10].
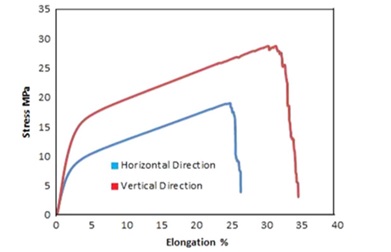
Figure 2. Tensile testing on cellular microstructure with 15% volume fraction and 3 mm unit cell size's stress-strain curves [10].
In a research study on 316L stainless steel, impact testing was utilized to examine its behavior under varying temperatures. Multiple impact tests were conducted at different temperatures, revealing the influence of temperature changes on both impact resistance and fracture energy. The Charpy test specimen was used to assess the impact of groove depth and angle, elucidating the material's formability behavior affected by temperature within the range of 20-300 °C. Furthermore, the investigation highlighted the significant impact of various geometrical features, such as the shape of the slit (U or V) and angle. Specifically, the sample with a U-shaped gap exhibited greater resistance compared to the sample with a V-shaped gap, maintaining consistency in temperature and thickness. Furthermore, within the V-shaped gap sample, an increase in the angle resulted in enhanced toughness [11].
Temperature and Strain Rate Effects on 316 Stainless Steel Fracture Behavior
A study looked at how sintered 316L stainless steel deformed and fractured under varied strain rates and relative porosity density circumstances [12]. The study involved fabricating samples of 316L stainless steel with varying relative sintered densities (83%, 88%, and 93%) and subjecting them to dynamic impact testing and low strain pressure tests with strain rates ranging from 3x103 to 9x103 (S-1) and 10-3 to 10-1 (S-1). The results revealed that both the relative density of porosity and the strain rate significantly influenced the mechanical behavior of the steel, particularly showing distinctions between low and high strain rates.
Increasing the strain rate and relative porosity density was found to enhance fracture stress while reducing the failure strain. Interestingly, the variation in strain rate had a more pronounced effect on stress and strain compared to changes in relative porosity. High strain rates caused samples to exhibit greater sensitivity to strain rate compared to those subjected to lower strain rates of 10-3 to 10-1 (S-1). Moreover, it was observed that elevating the strain rate while maintaining a constant relative density, or increasing the relative porosity density while keeping a consistent strain rate, amplified the sensitivity to strain rate. These findings also highlighted different mechanisms governing deformation at low and high strain rates.
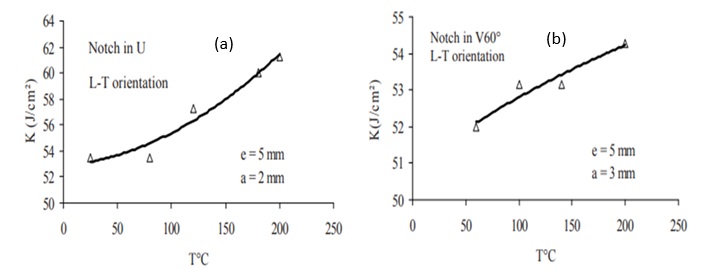
Figure 3. Impact resistance versus temperature: (a) V-shaped groove with an angle of 60 degrees and (b) U-shaped groove [11].
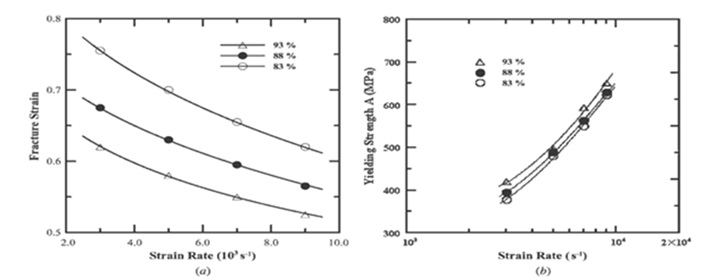
Figure 4. (a) Failure strain and strain rate relationships for various relative porosity densities and (b) Yield strength variation with strain rate for various relative porosity densities [12].
This study revealed a substantial correlation between relative porosity density, strain rate, and the volume of thermal activation. Factors such as work-hardening stress and thermal activation energy were closely linked to strain rate and the heat sensitivity of the activation volume. Microstructure analysis indicated that under constant strain rates, slip bands within deformed grains became more pronounced with increased strain rates for a given relative density, showcasing varying degrees of microhardness.
Moreover, the study found that stress concentration facilitated the formation of slip bands near grains and micropores. It was noted that grain boundaries were sites where secondary phase particles of diverse sizes developed.
The fracture analysis provided insights into the qualitative understanding of how porous 316L stainless steel fractures. The primary mechanism of failure involved significant localized shear cuts leading to the emergence of shear bands due to localization and stress gradients. The fractures propagation inside these shear bands resulted in sample failure. Notably, samples with high relative porosity density and high strain rates exhibited more prominent shear band development and cracking due to the higher degree of grain deformation caused by increased strain rate sensitivity.
The fracture characteristics showcased evidence of a softer failure, evident in the presence of dimple-like structures with varying depths and densities. These fracture characteristics trended towards softer fractures with decreasing strain rate or relative density of porosity [12].
In this investigation, the impact of temperature and strain rate on the tensile deformation and fracture behavior of 316L (N) austenitic stainless steel was explored. Various parameters like stress changes, tensile strength, and ductility values were measured using metrics such as uniform elongation, elongation to break, and decrease in cross-sectional area across different temperature ranges. Distinctive behavior was observed in three separate temperature ranges. When the temperature was varied from 300 K (27 °C) to 523 K (250 °C), it resulted in marked alterations in stress and strength data. Higher temperatures led to a rapid decline in strength while exhibiting a substantial increase in tensile ductility after reaching its minimum at room temperature. At moderate temperatures, the steel exhibited microstructures distinct from those observed at lower and higher temperatures. The research highlighted the significant impact of negative strain rate sensitivity on values such as stress-strength, average work-hardening rate, and minimum ductility at an average temperature. Across all test conditions, the steel consistently displayed an intergranular fracture pattern.
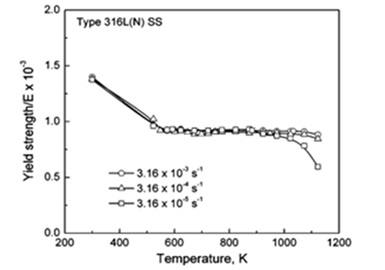
Figure 5. Yield strength variations with temperature and various strain rate [13].
Decreasing strain rates and increasing stress-strength and work-hardening rates were associated with a reduced likelihood of dendritic formations, peaks, and dimples at lower temperatures. Conversely, higher temperatures showcased a rapid decline in work-hardening rate and stress-strength values, coupled with increased ductility as the temperature rose and the strain rate decreased. These observations underscore the dominance of dynamic recovery mechanisms [13].
The Effect of Hydrogen and Helium on the 316 Steel's Fracture Properties
The physical differences between the tensile tests of austenitic stainless steels 304 and 316 conducted in the presence of internal and external hydrogen were assessed by a research [14]. Hydrogen-induced stress concentration and its role in aiding crack propagation significantly influence hydrogen-induced fracture in various settings. The presence of hydrogen often triggers a localized fracture process, leading to the expansion of stress-sensitive material fissures that compromise the tensile strength of the material. Alloys with lower nickel content and exposure to lower temperatures tend to be more vulnerable to hydrogen-induced fracture propagation.
The study also uncovered differences between the impacts of external and internal hydrogen on tensile ductility. Internally embedded hydrogen tends to result in less severe surface cracking and can impede hydrogen-induced fracture propagation from the material's surface. Conversely, external hydrogen exposure promotes the development and propagation of cracks through the material's surface. However, the degree of ductility loss caused by internal versus external hydrogen varies depending on specific test conditions, including hydrogen gas pressure. Furthermore, internal hydrogen may contribute to the formation of internal fractures, which possess a similar potential for propagation as surface cracks [14].
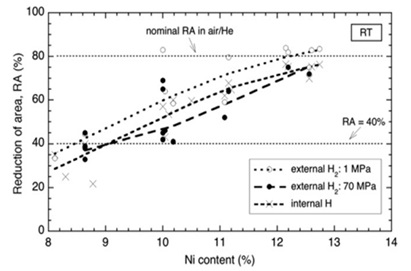
Figure 6. Reduction in cross-sectional area for the tensile test at room temperature as a function of nickel concentration. The measurement was performed using hydrogen gas at a pressure of 70 MPa [14].
The study findings indicated that observed patterns remained consistent regardless of hydrogen gas pressure (maintained at a minimum of 1 MPa) and whether trials were conducted with or without external or internal hydrogen. Notably, high-pressure external hydrogen led to a more significant decline in tensile ductility compared to internal hydrogen, as observed within the parameters of this study. The observed variation in tensile ductility can be attributed to hydrogen-assisted fracture growth. Materials inherently susceptible to hydrogen exposure may develop internal cracks, contributing to potential material failure. Tensile strength serves as an indicator to assess the extent of these processes. The study revealed that hydrogen-induced fracture in austenitic stainless steels, during tensile testing, involves a competition between stress concentration and hydrogen-induced crack propagation. Tensile strength diminishes when hydrogen-induced fracture propagation takes precedence. However, in hydrogen-rich environments where failure is primarily due to stress concentration, tensile strength does not decrease [7]. Another study examined the effects of alloy composition and strain hardening on tensile fracture in pre-charged 316 stainless steels [8]. The research identified that a 136 vppm internal hydrogen concentration, generated through thermal pre-charging of hydrogen gas, led to reduced tensile ductility in 316 stainless steel. However, both annealed samples and strain-hardened microstructures showed minimal loss in ductility despite this hydrogen concentration. The relatively high ductility observed in both hydrogen-pre-charged and non-hydrogen-charged samples suggests the involvement of plasticity processes in the types of tensile failure observed. The presence of internal hydrogen was found to either reduce nucleation sites or promote localized slips, strengthening interparticle bonds. Nickel's significance in deformation processes appeared crucial, as higher nickel content in 316 stainless steels seemed to offer increased resistance to hydrogen-induced fracture. Furtehrmore, while carbon was anticipated to contribute to the stability of 316 stainless steel due to strain-induced martensite formation, it was observed to have no discernible impact on hydrogen-induced fracture [15].
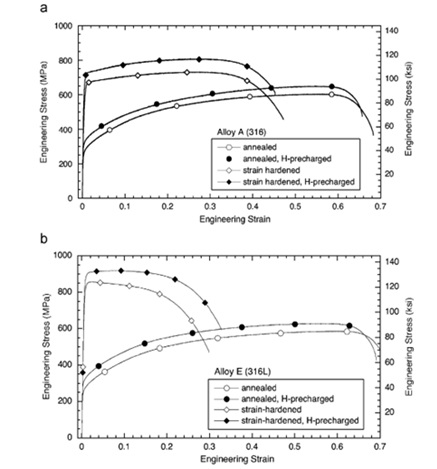
Figure 7. Tensile curves in annealed conditions and strain hardened in conditions without hydrogen and pre-charged hydrogen [15].
The study findings revealed that hydrogen dissolution did not enhance the alloying composition or microstructure of 316 stainless steel. Notably, reductions in ductility were observed in both annealed and strain-hardened microstructures of 316 stainless steel featuring an average internal hydrogen concentration of 136 wppm. In alloys containing internal hydrogen, a significant area loss exceeding 47% indicated a reduction in ductility ranging from 43% to 12%. Notably, alloys with lower nickel content exhibited the lowest ductility. Comparison of the cross-section area decrease between strain-hardened microstructures and their annealed counterparts, both with and without internal hydrogen, revealed only slight variations. Fracture surface analysis of both hydrogen-free and hydrogen-pre-charged phases displayed indications of micro-hole merging and joining. The size of dimples on fracture surfaces decreased with increased internal hydrogen concentration. In addition, fracture surfaces of materials containing internal hydrogen, particularly those with low nickel contents, exhibited features associated with slip bands or stress-related fractures due to deformation of the martensite phase. The quantity of nickel significantly influenced the resistance of 316 stainless steel to hydrogen-induced fracture, with more noticeable ductility loss observed for compositions containing less than 12% nickel. Increased nickel content contributed to enhanced stability of austenite and increased energy of stacking faults, thereby boosting the material's resistance to hydrogen-induced fracture. Interestingly, variations in the proportion of carbon within the range of 0.043% to 0.014% had no discernible impact on the fracture behavior of these alloys in the presence of hydrogen [15].
Another investigation [16] used high voltage electron microscopy (HVEM) analysis to look at how hydrogen affected the deformation and fracture of 316 austenitic stainless steel. The study's findings are listed below.
- The research discovered that austenite phase change and dislocation propagation from the fracture tip were related to the emergence of intergranular cracks in both vacuum and hydrogen environment settings. Hydrogen's main effects on fracture processes included lowering the stress necessary for dislocation displacement, crack propagation, and transformation of the gamma phase with FCC (face-centered cubic) structure into martensite phase with HCP (hexagonal close-packed) structure and martensite with BCC (body-centered cubic) structure. 2. In both non-sensitive and sensitive stainless steels, hydrogen-induced intergranular fracture was discovered to be accompanied by an increase in local plasticity at the front of a fixed crack, followed by a narrowing of the primary crack hole in one area. The crystalline background of the FCC structure and the hole's development were connected.
- Along the fracture surfaces and in front of the crack tip, martensite phases with ε and α ́ structures were found to occur, according to the research. It was discovered that cracks spread along the active slip planes and into the ε martensite phase. On the other hand, the α ́ phase was consistently seen in high-stress areas [16].
Another study investigated hydrogen-induced fracture in 316L stainless steel samples with primary cracks [17]. Tensile testing conducted on 316L austenitic stainless steel specimens, both with and without concurrent cathodic polarization, revealed that hydrogen-induced damage did not significantly alter the macroscopic failure mechanism of the specimens when subjected to slow loading. Under these test conditions, hydrogen damage manifested as cracking phenomena restricted to the fracture root, accelerating rupture by generating additional branches from the original break.
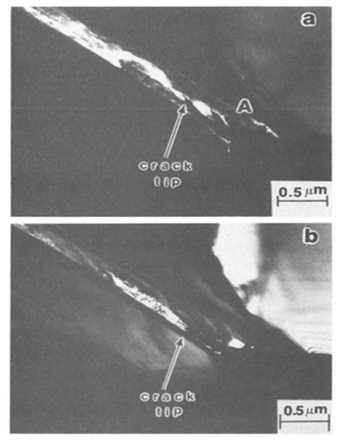
Figure 8. Strained in a vacuum, the sensitized steel exhibits martensitic phases in an intergranular fracture. The dark field images are related to (a) diffraction spots of martensite ε and (b) martensite α ́ [16].
Despite the growing impact of hydrogen at the applied loading rate, the steel demonstrated a high degree of resistance to defects, as substantial damage was required for failure to propagate. A novel damage phenomenon, involving a bifurcated crack, emerged under conditions of high stress concentration, such as a crack, combined with an extremely low loading rate. In such cases, the samples experienced a soft failure after accumulating significant damage, yet the geometric modifications induced by this additional hydrogen action expedited plastic rupture. It was suggested that hydrogen action, when rapid, competed with mechanical deformation and emerged as a secondary dominant mechanism, potentially explaining variations in damage phenomenology and their dependence on loading rates.
However, this process was restrained by the convergence of the crack extension with the crack tip, propagating at a rate below the critical limit, resulting in a dominant damage mechanism. Initially, hydrogen tended to generate and propagate a bifurcated crack at the fracture tip [17].
Investigations were also done on how helium affected the stress fracture behavior of 316 stainless steel [18]. These are the findings of this research study: 1. The presence of helium in 316 stainless steel leads to a reduction in both the elongation rate and the material's rupture life.
- In the dissolved and aged annealed state of 316 stainless steel, where austenitic grains contain M23C6 at grain boundaries, the rupture life can decrease for up to 1000 hours based on the helium concentration, ranging from 1.5 x 10-6 to 4 x 10-5 atoms.
- Under quenched and aged working conditions of 316 stainless steel, the reduction in fracture life correlates with the helium concentration in the material. The presence of sigma phase dispersed in the matrix for the quenched working sample impacts this reduction. For samples starting with an atomic fraction of 1.5 x 10-6 helium, the decrease in rupture life rises with decreased starting stress. Meanwhile, for samples with a helium atomic fraction of 4x10-5, the drop in rupture life remains relatively constant until around 300 hours, after which the alloy experiences a significant loss in creep strength.
- The strain observed within the grains of samples containing helium represents a minimal fraction of the total length increase observed post-testing. Intergranular cracking emerges as the primary cause of the observed elongation [18].
Heat Treatment's Effect on 316 Steel's Fracture Characteristics
The effect of grain modification and heat treatment on fracture characteristics caused by hydrogen in 316 stainless steel was also investigated by a team [19], and the following results were obtained. 1. Unmodified steel generally exhibits lower toughness properties compared to modified steel. This disparity often arises from the larger grain size present in unmodified steel in contrast to the smaller grains found in modified steel. 2. Hydrogen embrittlement tends to be more pronounced in structures characterized by larger grains and higher dissolution annealing temperatures.
- The modification process in steel can lead to severe hydrogen embrittlement due to the reduction of chromium along grain boundaries. 4. Observations suggest the absence of detectable α ́ martensite in 316 stainless steel subsequent to hydrogen charging [19].
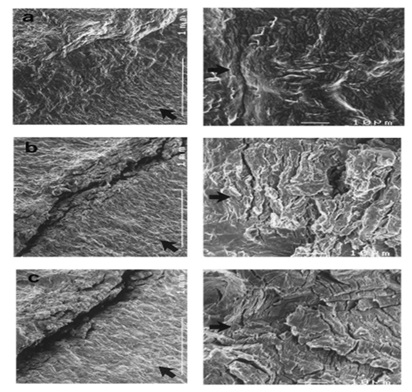
Figure 9. Fracture tip as shown in SEM photos just before maximum loading: (a) hydrogen-free and (b,c) under hydrogen charging at 10 and 1 mm/min, respectively [17].
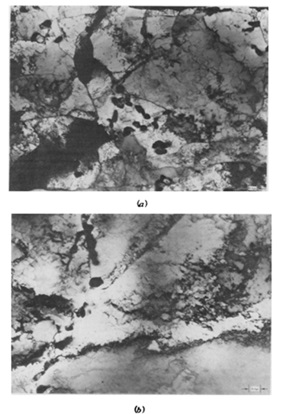
Figure 10. SEM images of samples heat treated at 700 ⃘C. (a) Base sample, rupture life = 1075 h, elongation = 44% and (b) Helium fraction = 4 x 10-5 atom, rupture life = 316 h, and elongation = 13% [18].
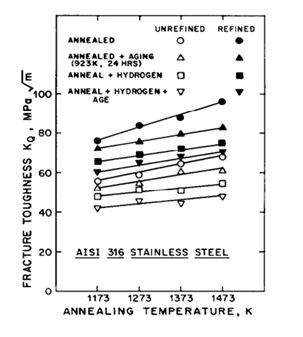
Figure 11. Fracture toughness diagram according to annealing temperature [19].
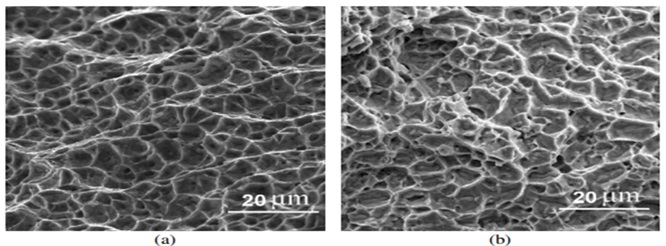
Figure 12. Dimples on the weld metal fracture surface as seen in SEM photos (a) without aging and (b) aged with shear fracture mode [20].
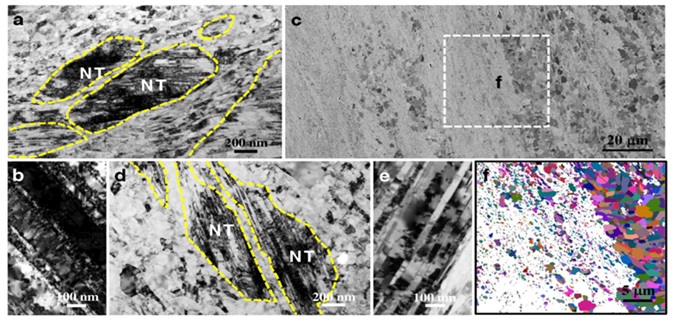
Figure 13. (a) A nanograin structure with nanotwin (NT) bands is seen in the cross-section microstructure of 316L steel, (b) a TEM picture of twins under strain that has a lot of accumulated dislocations, (c) a cross-section SEM picture of the sample's microstructure after it was annealed at 720 °C reveals some grains that have recrystallized, (d) a closer TEM study of the undissolved portions in (c) reveals nanotwin bands encircled by coaxial grains that are less than one micron in size, (e) TEM picture of nanotwins that have been annealed and have a low dislocation density, and (f) an EBSD map of the area in (c) that was chosen, showing the presence of high-angle grain boundaries and grains that have recrystallized with annealing twins [1].
Another study [20] looked at how 316L stainless steel weld metal's fracture and room- and high-temperature tensile characteristics were affected by aging. The following are the findings of this research:
- The brittle sigma phase particles quickly replace the delta ferrite phase when aging 316 stainless steel at temperatures of 750 °C and 850 °C. Tensile strength just slightly rises as a consequence, while tensile ductility significantly falls.
- The shear mode predominates at a tensile test temperature of 500 °C, and the flat mode is generally the macroscopic failure mode in welded metals. The metal from the weld, however, may have a cup-cone fracture surface.
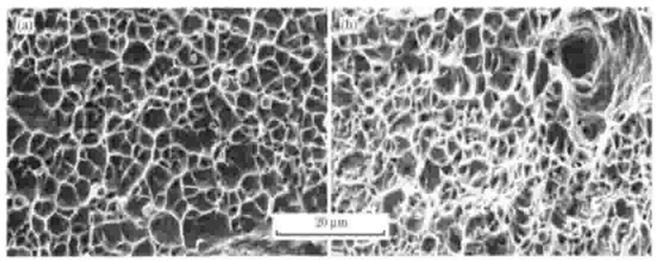
Figure 14. SEM images of fracture morphology: (a) heated rolled sample and (b) dissolved annealed sample [21].
- The shear fracture mode in welding metals causes a local deformation along the sub-borders of primary cavities, which causes cracks in the sigma phase particles with an orientation of about 45 degrees in the loading direction.
- The change of fracture mode from inclined to flat occurs at room temperature, which seems related to increased strain hardness with time and temperature [20]. In one investigation, 316L austenitic stainless steel with a heterogeneous distribution of nanotwin bundles produced by dynamic plastic deformation and annealing at various temperatures was tested for fracture behavior [1]. The findings demonstrated that controlled annealing of 316L austenitic stainless steel reduced strength somewhat while dramatically increasing fracture resistance, enhancing the synergy of strength and toughness. The production of recovered micron-sized grains and recrystallization, which postponed the development of fractures, was credited with this increase in toughness. In addition, the remaining nanotwin bundles helped to stop crack growth [1]. In another study [21], mechanical characteristics and microstructure for austenitic stainless steel 316L were assessed in two treatment modes, dissolved annealing, and hot rolling, and the upcoming results were obtained.
- The strength and elongation of the solution-annealed sample were found to be better than those of the hot-rolled sample. The higher density and depth of the dimples observed in the annealed sample can explain its better elongation. Furthermore, the amount of ferrite decreased from 10% to 6% after the dissolution treatment, which contributed to improved corrosion properties.
- 316L steel is known to be easily work-hardened during deformation. The work hardening rate curve in terms of true strain shows that the dissolved work sample exhibits a more uniform length increase than the hot-rolled sample. This is because the work hardening rate of the dissolved work sample decreases more slowly than that of the hot-rolled sample.
- The mechanism of twin formation in 316L steel can be divided into two categories: the movement of partial dislocations and the accumulation fault mechanism, the latter of which occurs in the case of intergranular twins.
- Deformation can cause martensitic transformation in 316L steel, making it susceptible to α ́ martensite formation. The strain that occurs at the shear band intersections is responsible for the formation of α ́ martensite, which can be detected through XRD analysis [21].
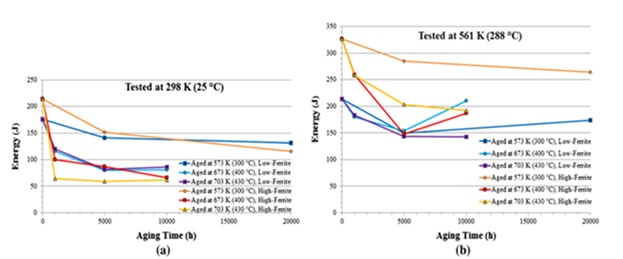
Figure 15. Results of the Charpy V-notch impact toughness test are provided for materials with low and high delta ferrite values (a) at 298 K (25 °C) and (b) at 561 K (288 °C) [22].
Furthermore, it has been investigated how aging affects the stress corrosion cracking (SCC) and fracture toughness of 316L weld metal [22]. The findings of this investigation are as follows:
- Nano topography allowed researchers to see the hardening of delta ferrite. This phenomena was seen at 573 K (300 °C), and it was most likely caused by the spinodal breakdown of the delta ferrite phase. Significant G phase precipitation occurs inside the delta ferrite phase as well as along dislocation lines at temperatures as high as 673 K (400 °C).
- Due to the low carbon concentration of the material, M23C6 carbides did not seem to be deposited at the borders between the austenite and delta ferrite phases in the weld metal.
- As aging time and temperature rise, the impact energy shown in the Charpy V-notch test falls until it approaches saturation. The delta ferrite phase experiences brittle failure after saturation. The quantity of ferrite phase present, the test temperature, and the aging temperature are some examples of variables that affect the saturation of impact toughness.
- 316L weld metal has a greater stress corrosion cracking (SCC) crack propagation rate than wrought (worked) metal. In particular, the weld metal aged at 673 K (400 °C) for 5000 hours had an increase in the SCC crack development rate that was almost double that of the unaged sample.
- In 316L steel, SCC crack development often occurs at the junction of the austenite and delta ferrite phases. The delta ferrite and austenite phase border cracks during the crack propagation process, and then slower cracks in the austenite phase develop between dendrites.
- When tested at 561 K (288 °C) in a high-purity water environment with 300 ppb of dissolved oxygen, the in situ fracture toughness of a material is much lower than the results obtained when testing in air at the same temperature. In addition to a decrease in toughness, the environment may also cause a drop in the rupture (tear) modulus.
- Intergranular brittle fracture was observable on the fracture surfaces during the fracture toughness testing of the materials in the high-purity aqueous environment with dissolved oxygen; however, this was not the case for the samples tested in air. In comparison to samples examined in air, the fracture path of the tested samples seemed smoother, and the quantity of shrinking was noticeably less. 8. According to this study, the increased lattice mismatch between the austenite and delta ferrite phases is mostly responsible for the rise in the SCC crack development rate during age. The crack growth rate is more influenced by this component than by any other microstructural feature. At high temperatures in an aqueous environment, the presence of hydrogen may also weaken the material's resistance to fracture. At the fracture tip and on other sample surfaces, hydrogen may form, and both permeable and trapped hydrogen may be a factor in this problem [22].
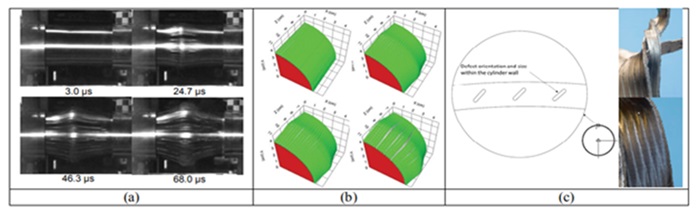
Figure 16. (a) Images captured by the Phantom V1610 camera show the expansion and failure of an AM stainless steel cylinder that contained defects created during the manufacturing process. The times given are according to the impact time. The projectile hits the middle point from the left side. The cutting area can be seen as strips along the length of the cylinder, (b) 3D simulations completed in CTH, and (c) images of the recovered parts show the strain's location along the defect plane [23].
Fracture Behavior of 316 Alloy under Different Operational Parameters
Investigations have been done into the fracture properties of 316L stainless steel manufactured with additives or cylinders of the same steel made from wrought iron rods and put under stress [23]. The gas gun approach for expanding cylinder testing makes it possible to evaluate the ductility of metals as well as the processes of corrosion and fracture that take place during the high-speed tensile test. Through these tests, the strain rate may change from 10-2 to 10-4 (S-1). The findings showed that, in the examined strain rate range, an additively manufactured cylinder failed at a greater strain than cylinders produced of forged rods.
Furthermore, the effect of macroscopic holes put at a 45 degree angle to the cylinder radius was examined. Results from simulations and experiments (using the Eulerian hydrocode CTH) were contrasted as well. Four 316L stainless steel cylinders were tested to failure, and the strain-to-failure values (a measure of ductility) showed that the AM cylinder (manufactured by additive material technique) was more ductile than its conventional counterpart. It is suggested that this change in ductility was due to the preferential orientation of the grains formed during the AM fabrication process. The orientation control of the defect site or locations was
achieved by determining the macroscopic defects in the cylinder wall. If there are changes in the microstructure, a restoration technique should be used in the future. Simulations using the CTH hydrocode initially showed reasonable compliance, but deviations from the experimental data were observed with the onset of failure. Future work should focus on the AM technique's effect on the material's final microstructure, which seems to play an essential role in the failure mechanisms. Introducing defects of different sizes using material addition techniques can enable an understanding of how the fracture patterns and distribution of defects in a material are related. Multiple-impact velocities should be used to determine the influence of strain rate on ductility. Probably, this action leads to the development of a strain-rate-dependent failure model. Expanding rings instead of cylinders means uniaxial stress conditions exist throughout the experiment, leading to more superficial data interpretation [23].
Another study looked at the connection between the 316L stainless steels totally austenitic microstructure and fracture behavior in welded joints [24]. In comparison to standard 316L filler material, the fracture toughness of multi-pass weld deposits made using Mn-modified 316L filler material (4-5 wt%) was found to be less than 100 (MPa.√m). Although gentle rupture causes failure in both materials, the fracture route varies. The results display the "corduroy" morphological fracture surface. Surfaces in samples under strain and compression had corresponding cracks that revealed a sparse amount of typical 316L weld deposits. The fracture morphology and toughness of welded joints may be connected to the microstructural stability of austenite during testing, according to a suggested model. Manganese and molybdenum that precipitate at cell dendritic borders during weld solidification have a tendency to stabilize the austenite and stop martensite from forming there. Therefore, in Mn-modified weld deposits, fracture preferentially occurs at these boundaries, leading in a "corduroy" fracture morphology and poorer fracture resistance than in weld deposits where martensite formation is more homogenous [24].
In both conventional and Mn-modified 316L weld deposits, scanning electron microscopy investigation showed the development of molybdenum- and manganese-rich structures along the solidification boundaries. It was essentially because of the extra Mn in the changed electrodes that the Mn-treated material displayed more noticeable elemental enrichment at the borders. Crack propagation favored the boundaries of the substructure, as seen by fracture surface profiles and fracture fractography of tensile and compression specimens of 316L weld deposits with Mn modifications.
A suggested model connects the microstructural heterogeneity brought on by element partitioning during solidification to the variations in fracture toughness of completely austenitic 316L weld deposits. Austenite around the solidification boundaries is more stable than the surrounding microstructure at cryogenic temperatures and does not change into martensite. Incomplete transformation in Mn-modified welding deposits results in a particular microstructure-based fracture morphology and lowers fracture resistance. However, increasing the stable austenite at the borders or getting closer to the bulk microstructure may be achieved by lowering the partitioning of alloy elements along the boundaries, whether by altering the filler material composition or the welding circumstances. This causes austenite to convert more completely into martensite, increasing the weld microstructure's resistance to fracture [24].
In another study [25], the fracture mechanics caused by stress corrosion cracking (SCC) in austenitic 316 steel work-hardened in hot MgCI2 aqueous solutions was investigated, and the following results were obtained:
- The stress intensity is minimal when the SCC speed is less than KISCC≈12 MN.m-2/3.
- When the value of KI was increased, three consecutive regions of K1-ν behavior were observed. In region I, the KI behavior was dependent on the rate. In region II, its rate remained constant and was independent of the KI value. This was followed by region III, where the value of ν was again observed to be dependent on KI.
- The range of the apparent activation energy for the behavior observed in region II is between 63 and 67 kJ/mol.
- The intergranular and intragranular relative relationships are significantly influenced by the values of KI and electrochemical potential E. Intergranular stress corrosion cracking (SCC) was found to boost with enhancing KI, regardless of the value of E. Additionally, intergranular SCC also increased with increasingly negative (active) values of E, regardless of the KI value.
- Based on the calculation of corrosion potential and corrosion products, the pH value is less than 1 when the crack propagates under free corrosion conditions. It increases to about 4.5 when the crack stops.
- The findings were in line with the plasticity effects observed at the crack tip, which lead to the rupture of passive layers, exposing the metal surface during SCC. Several aspects of region II were consistent with an SCC mechanism that involves controlled adsorption processes on the metal surface or controlled hydrogen penetration into the lattice near the crack tip [25].
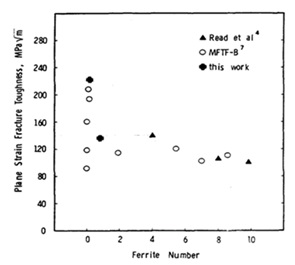
Figure 17. The influence of the amount of ferrite phase in weld on the fracture toughness of 316L weld [24].
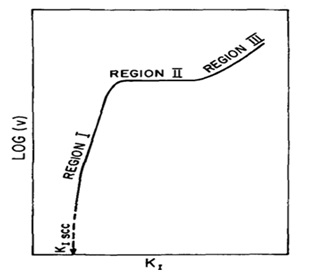
Figure 18. Schematic behavior (KI-logν) for SCC [18].
In another investigation [26], the fracture toughness of two kinds of 316LN steel welded joints was assessed at 300 and 623 K in air in various directions. The tests were carried out using the single sample approach, where fracture toughness values (J0.2) and J-R curves are computed. Before and after the test, the samples' microstructure was assessed. According to the findings, the 2GT welded junction is more durable than the 5GT welded joint, which has minimal bearing on fracture toughness. In addition, when temperature rises, fracture toughness falls significantly. The findings of this study are outlined as follows:
- The material's fracture toughness drops by about 0.45 between 300 K and 623 K. According to the fracture morphology, the early-stage fractures are mostly intergranular. Material failure after the tensile stage is mostly plastic deformation failure.
- The EBSD result demonstrates the formation of ferrite and austenite grains in the direction of the crack's propagation. 4. The distribution maps of the Taylor factor showed that the 2GT sample had a higher Taylor factor than the 5GT sample, indicating a higher plastic strength for the 2GT sample. This observation is in line with the experimental results [26].
In another study [27], software was used to predict soft fractures in 316LN stainless steel during hot deformation. The following outcomes were found:
- Based on the findings of the tensile tests, a model was created to estimate the material's critical failure strain as a function of temperature and strain rate.
- The 316LN stainless steel soft fracture criteria takes into consideration plastic effects, the material's ability for deformation, and the stress condition. The Ovian criteria and a critical failure strain model serve as the foundation for this criterion.
- To allow the prediction of crack start and placement, the proposed soft fracture criteria was included into the commercial DEFORM-2D program. The established criteria was able to precisely predict the onset and location of fractures at high temperatures, as shown by the comparison of the simulated and experimental findings [27].
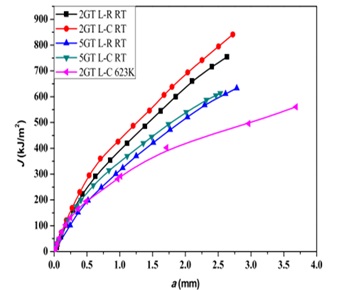
Figure 19. J-R diagrams for 2GT and 5GT connections [26].
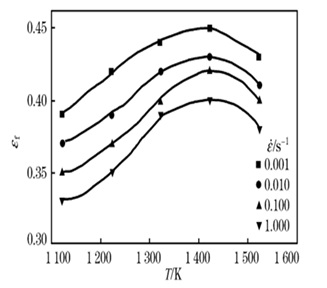
Figure 20. Values of the critical strain of failure (εf) at different strain rates and temperatures [27].
Conclusion
- In 316 SS, a strong correlation exists between its microstructure and mechanical properties like yield stress and fracture topography. Both the Hall-Petch equation and the Gibson-Ashby micromechanical model prove useful in predicting these mechanical properties.
- Deformation strain-rate holds greater significance than relative porosity density. At a constant strain rate, increased relative density correlates with a higher count of slip bands and grain deformation. Observations of shear bands indicate local shear as the predominant form of fracture.
- Hydrogen's impact on the environment is not an immediate process; however, it acts as the secondary dominant deformation mechanism. While hydrogen damage doesn't alter failure modes at low strain rates, its primary influence lies in reducing the stress required for dislocation displacement and crack propagation. Consequently, the presence of hydrogen decreases tensile strength. External hydrogen, in some cases, exhibits a more significant effect.
- In quenched and aged 316 SS, a correlation exists between helium concentration and reduction in fracture life.
- Heat treatment processes significantly affect the ferrite phase and its interface with the cementite phase in 316 SS. Aging, for instance, increases lattice mismatch in delta ferrite and austenite phases, influencing fracture morphology at high temperatures. Controlled annealing can enhance fracture resistance but may decrease strength. Careful execution is vital as reducing grain boundary chromium heightens the risk of hydrogen embrittlement. Exploring novel heat treatment methods aimed at optimizing fracture resistance without compromising strength in 316 SS could be a promising area of investigation. Investigating alternative alloy modifications to mitigate hydrogen embrittlement effects, considering the delicate balance between microstructure alterations and mechanical properties could also be a valuable avenue for further research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Orcid
Mohammad Sajjadnejad : 0000-0001-5112-1791
Yashar Behnamian : 0000-0002-7313-4021


