Document Type : Original Research Article
Authors
- Nur Shazwani Binti Abdul Latif 1
- Wan Md Zin Wan Yunus 1, 2
- Hafizah Binti Ariff 1
- Rauda Binti A. Mohamed 3
- Nurnadia Binti Andenan 4
- Khoirul Solehah Binti Abdul Rahim 1
- Noor Azilah Binti Mohd Kasim 5
- Keat Khim Ong 5
- Alinda Binti Samsuri 1, 5
1 Centre for Tropicalisation, Defence Research Institute, Universiti Pertahanan Nasional Malaysia (UPNM), 57000 Sungai Besi, Kuala Lumpur, Malaysia
2 Faculty of Defence Science and Technology, Universiti Pertahanan Nasional Malaysia (UPNM), 57000 Sungai Besi, Kuala Lumpur, Malaysia
3 School of Applied Sciences, Nilai University, 71800, Nilai, Negeri Sembilan, Malaysia
4 Centre for Research and Innovative Management, Universiti Pertahanan Nasional Malaysia (UPNM), 57000 Sungai Besi, Kuala Lumpur, Malaysia
5 Centre for Defence Foundation Studies, Universiti Pertahanan Nasional Malaysia (UPNM), 57000 Sungai Besi, Kuala Lumpur, Malaysia
Abstract
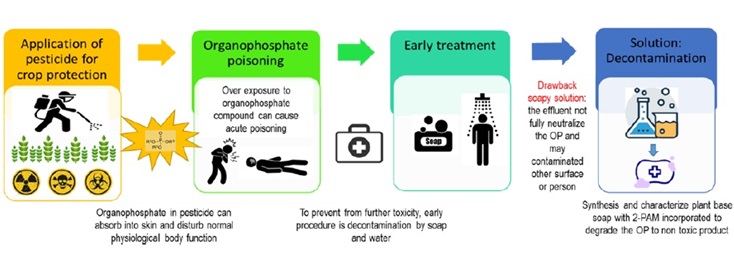
Extensive utilization of pesticides in the agricultural sector is acknowledged for their potent insecticidal properties, effectively safeguarding crops and consequently enhancing both yield and quality. However, the organophosphate compounds (OPs) predominantly found in these pesticides are highly toxic and known for their rapid skin absorption, leading to acute poisoning and posing public health challenges. Early exposure requires immediate and efficient decontamination to prevent further skin absorption of OPs. The current cleaning agents, such as soap and bleach, are often associated with harsh chemicals that can be corrosive to the skin. Furthermore, these conventional options tend to merely wash away chemicals, potentially leaving effluent that still carries toxic compounds, causing pollution to the environment. Thus, there is a need for approaches that not only clean effectively but also degrade the harmful substances. This paper proposes a method to synthesize solid soap from palm oil incorporating 2-PAM as an active agent for effective decontamination. The resulting yellowish solid soaps exhibit pH values in the 9-10 range, lathering capabilities, and controlled moisture content (4.19 % for S1, 2.42 % for S2, 2.1 % for S3). FTIR analysis confirms successful 2-PAM integration in soaps S2 and S3, as evidenced by specific bands at 3016 cm-1 to 3015 cm-1, 1736 cm-1 to 1735 cm-1, 1368 cm-1 to 1364 cm-1, and 1217 cm-1 to 1215 cm-1. These characteristics set the stage for further research into the efficacy of these soaps in organophosphate decontamination and their role in environmental pollution control.
Graphical Abstract
Keywords
Main Subjects
Introduction
It is predicted that by the year 2050 the global food production will need to increase by 70 % to meet the demand resulting from the growth in population [1]. Consequently, numerous initiatives have been undertaken to enhance food production and supply [2,3]. One approach to improve crop quality and yield is the use of pesticides for crop protection [4,5]. The utilization of organophosphate compounds (OPs) as pesticides is favored over organochlorine due to their lower persistence in the environment and the absence of toxic metabolites [6]. Approximately two million tonnes of pesticides are used annually worldwide, with major contribution from three countries: China, USA, and Argentina [7]. These nations possess large and diverse agricultural sectors where pesticides are commonly employed to safeguard crops from pests, diseases, and other threats. The significant pesticide usage in these countries reflects the scale of agricultural activities and underscores the importance placed on crop protection to ensure food security and economic stability.
The importance of OPs in preventing crop losses is undeniable. However, numerous incidents of improper handlings and misuse of these chemicals have resulted in disastrous effects on the environment and human safety [8–11].
Pesticides can cause long-lasting harm to the air, water, soil, and living organisms due to specific traits, such as high lipophilicity, bio-accumulation, extensive half-life, non-biodegradability, and widespread dispersion [12,13]. Furthermore, the increasing resistance observed in pests, such as Spodoptera littoralis, to commonly used pesticides adds an additional layer of complexity [14]. This resistance issue intensifies concerns about the accumulation of pesticides, contributing to a more challenging scenario for environmental and ecosystem health.
Accidental pesticide poisonings resulting from skin exposure are a major cause of toxicity from OPs. The acute toxic effects of OPs, whether through ingestion, dermal contact, or inhalation, manifest as the inhibition of acetylcholinesterase (AChE). This inhibition prevents the acetylcholine hydrolysis, leading to its accumulation in nerve synapse and the subsequent development of intoxication symptoms. If not treated promptly, these symptoms can aggravate to fatality [15-17]. Thus, in the context of exposure incidents, the initial step in management involves efficient decontamination of chemicals from all surfaces to prevent them from their further infiltration into the body system [18]. Moreover, understanding whether contamination is a one-time occurrence or recurrent is crucial in decontamination, as it directly impacts the effectiveness of the decontamination strategies [19].
Effective decontamination involves the elimination or decomposition of toxic and dangerous substances from individuals, equipment and the environment, transforming them into harmless products [20]. Decontamination can be achieved through simple procedures, such as washing with soap and water, or using advanced decontamination products with absorption and agent degradation properties [21]. However, concerns arise regarding the use of soapy solutions, as they may only remove and dilute contaminants without fully neutralizing them, potentially leading to contamination in other areas or affecting additional victims [22].
Perhaps, the OPs exhibit slow biodegradation or non-biodegradability, giving rise to a new environmental challenge. If not promptly addressed, this issue can result in pollution and pose hazards to human health [23]. Consequently, efforts to improve the decontamination process have led to research on synthesizing decontamination agents that practical, easy to use, contain non-corrosive chemicals, contribute minimal toxicity, and remain stable for an extended period after production [24].
In medical field, oximes have been globally utilized as an antidote for the treatment organophosphate poisoning [25,26]. Oximes, such as pralidoxime and obidoxime, act as nucleophilic agents allowing the reactivation of acetylcholinesterase by removal of the phosphoryl group [27].
Moreover, various studies have further substantiated the potential of oximes as effective nucleophilic agents in neutralizing organophosphates [28–30]. Specifically, oximes containing pyridine derivatives have demonstrated the capability to be effective compounds in degrading harmful substances [31].
With the ability of the oximes to remove OP via nucleophilic attack at the phosphorus atom, leading to the cleavage of the P-O aryl bond [32], the addition of oximes such as 2-PAM to soap enhances the efficacy of the soap as decontamination agent against organophosphate. This report outlines the methodology for preparing the soaps and presents the results of their characterization, establishing the physicochemical properties prior to a study evaluating their efficacy in OPs decontamination.
Experimental
Materials
The palm oil utilized in this study was sourced from a local distributor in Kuala Lumpur, Malaysia, known as IKO Nature Sdn. Bhd. In addition, sodium hydroxide (anhydrous reagent grade ≥98% purity) and 2- Pyridinealdoxime methiodide (2-PAM) 99% purity were procured from Sigma Aldrich in the USA.
Preparation of soap
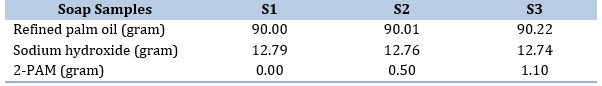
The preparation of solid soap containing 2-PAM involved a saponification process utilizing refined palm oil and sodium hydroxide. Initially, palm oil was mixed with a sodium hydroxide solution and an oxime solution (0.0 %, 0.5 %, and 1.0 % w/w). The mixture was stirred until it achieved a thick, creamy and smooth consistency. The resulting product was poured into soap molds and allowed to set for 24 hours at room temperature. This procedure was repeated for different concentrations of 2-PAM, as presented in Table 1. Following a curing period of six weeks, the soap underwent physicochemical testing to assess their qualities.
Table 1. Formulation of soaps
Physicochemical characterization of soaps
The physicochemical properties of all the prepared soaps were determined by using FTIR spectroscopy, pH measurement, foaming ability, and moisture content.
FTIR spectrometry
The sample was measured using Fourier Transform Infrared (FTIR) equipped with Attenuated Total Reflectance (ATR), obtaining spectra within the mid-infrared region (4000 to 650 cm-1) using Perkin Elmer Spectrum IR 10.6.1 and the FTIR data collection software, Spectrum IR version 10.6.1. Prior to each analysis and between each sample placement, the ATR plate was cleaned with acetone and soft tissue paper to avoid cross contamination [33,34].
pH analysis
The pH analysis of the soap samples was conducted at two distinct stages. The initial measurement was performed after 24 hours of saponification, and the second evaluation occurred after a six weeks curing period. One gram of the soap sample was dissolved in 100.00 ml of deionized water and heated to 70 °C [35]. The pH of the soap solution was recorded upon achieving complete dissolution.
Foaming ability test
The soap’s ability to generate foam, a crucial attribute for practical utility, was assessed using a modified method based on the work by Atolani [36]. For each solid soap sample, 0.2 g was dissolved in 10.00 ml of deionized water within a 100.00 mL measuring cylinder. After vigorous shaking for two minutes, the solution was allowed to stand for of ten minutes. The height of the resulting foam was then meticulously measured and recorded.
Moisture content assessment
Five grams of each soap sample were placed in an oven at a constant temperature of 105 °C until a consistent weight was achieved. The moisture content of each soap sample was then calculated as a percentage using the Equation 1. Equation 1: Calculation of solid soap moisture content
Moisture content (%) = (Loss in weight)/ (weight of sample) ⨉ 100 (1)
Results and discussion
As we explore the outcomes of this study, Figure 1 visually illustrates the soap samples (S1, S2, and S3), prepared according to the formulations outlined in Table 1. The initial soap sample, S1, exhibited a white color. However, the addition of 2-PAM gradually shifted the soap color to a creamy yellow, with the intensity of this coloration proportionally increasing as the concentration of 2-PAM increased.
Fourier Transform Infrared (FTIR) spectroscopy was employed to unravel the intricate molecular structures of palm oil and 2-PAM, which are key components crucial to our soap formulations. The FTIR spectra, along with the major functional groups in both palm oil and 2-PAM diligently detailed in Table 2, are illustrated in Figure 2 and Figure 3. This provides a comprehensive insight into their chemical compositions within the context this research.
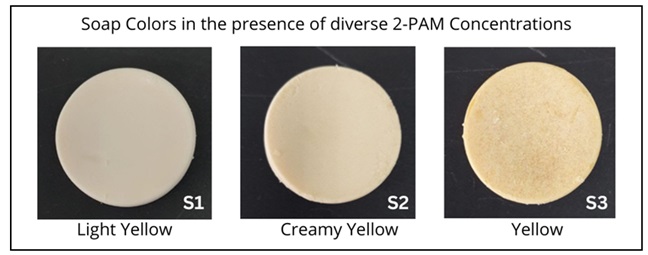
Figure 1. Changes in Soap Color with Different 2-PAM Levels.
Table 2. Table of major functional groups in palm oil and 2-PAM FTIR spectra
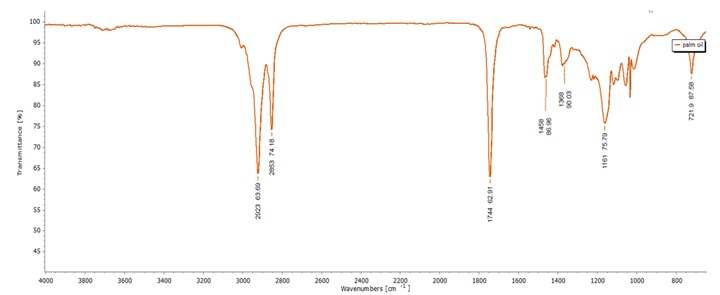
Figure 2. FTIR spectrum of palm oil
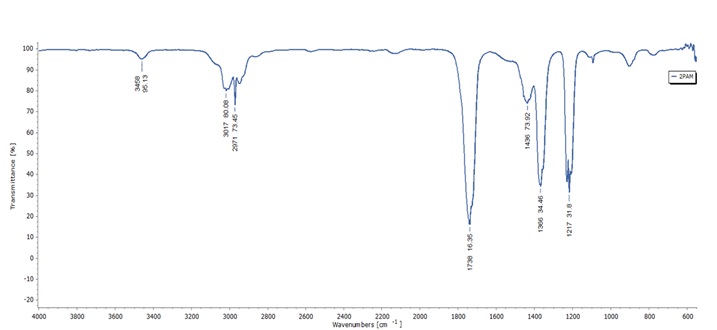
Figure 3. FTIR spectrum of 2-PAM
Palm Oil contains a functional group related to a (C=O) stretching band at a specific wavelength. Specifically, this frequency corresponds to the ester functional groups present in triglycerides, which are the major components of palm oil. Triglycerides consist of glycerol molecules esterified with three fatty acid chains, and the (C=O) stretching vibrations in ester groups characterize their structure [37]. In addition, palm oil exhibits vibrations related to the overlapping of methylene (-CH2) rocking vibrations at a frequency of 721 cm-1. This overlapping results from the complex structure of palm oil, with multiple methylene groups in its fatty acid chains [38]. Previous studies have reported a palm oil spectrum with comparable FTIR characteristics, confirming the presence of its primary functional groups [33,39].
As listed in Table 2, the results of the infrared (IR) spectrum of 2-PAM elucidate the chemical bonds within the compound. Notably, the IR spectrum of 2-PAM exhibits a distinctive weak band at 3458 cm-1, indicative of the O-H vibration associated with the –N=C group substituted in the pyridine ring. Additionally, two prominent bands are observed at 3017 cm-1and 2971 cm-1, corresponding to the asymmetric stretching vibrations of C-H bonds in methyl (-CH3) and methylene (-CH2) groups, as well as the pyridine ring. These band attributions are consistent with prior research by Manea [40].
Next, the FTIR spectra of the prepared soaps, S1 to S3, are illustrated in Figure 4. Both S2 and S3, showed similar characteristic bands at 3016 cm-1 and 3015 cm-1, attributed to the existence of C─H asymmetric stretching vibrations in CH3, CH═ and aromatic ring [41]. This peak closely resembles the one observed in the 2-PAM FTIR spectra. Additionally, bands at 1736 cm-1 to 1735 cm-1 for -C=N stretching [42], and 1368 cm-1 to 1364 cm-1, 1219 cm-1 to 1217 cm-1 for –C=C stretching [43], further confirm the 2-PAM presence within the soap formulation of S2 and S3. However, for S1 soap, no significant peaks were observed at 3011 cm-1, 1739 cm-1, or 1219 cm-1 signifying the absence of pyridine aromatic ring structure of 2-PAM in this soap.
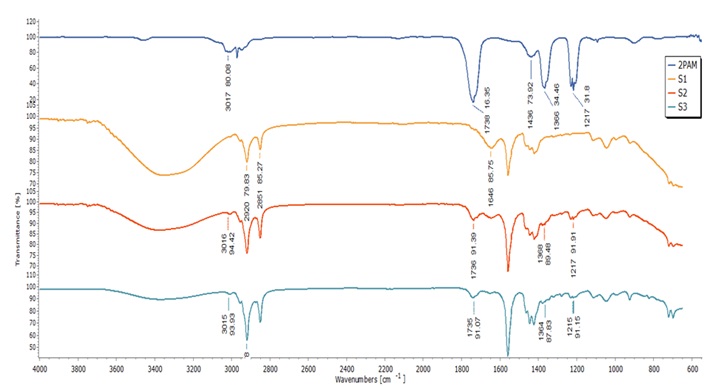
Figure 4. FTIR spectra of 2-PAM, S1, S2, and S3 soaps
Table 3 provides the physicochemical analysis results for the soap samples. The initial pH values of three different soaps (S1, S2, and S3) are presented both before and after the curing process. S1 (palm oil soap) displays a pre-curing pH of 11.12 ± 0.01, indicating strong alkalinity. After curing, the pH decreases to 10.16 ± 0.02. S2 (Palm oil + 0.5% 2-PAM soap) exhibits a pre-curing pH of 10.10 ± 0.04, and post-curing, the pH slightly decreases to 9.98 ± 0.02. S3 (Palm oil + 1.0% 2-PAM soap) starts with a pre-curing pH of 10.14 ± 0.07, and after curing, the pH is 9.54 ± 0.02. Higher pH values before curing are indicative of the incomplete hydrolysis of sodium hydroxide during the saponification process [44]. Typically, solid soap is considered ready for use after a curing process, which usually spans four to six weeks [45]. This curing duration promotes the evaporation of excess moisture from the soap, resulting in increased hardness, milder properties, and extended longevity. All the cured soaps exhibited pH values within the acceptable range of 9-10 [46].
Additionally, maintaining a slightly higher pH helps preserve the soap's integrity and prevent the growth of bacteria and mold. Soap with pH above 10 is associated with harshness on the skin [47]. In this study, all the pH values of the prepared soaps are within the recommended range, mildly alkaline, contributing to effective cleansing and removal of dirt and oils from the skin.
The foaming capacity of solid soap plays a pivotal role in its efficacy in cleaning and lathering. Excessive, voluminous foam can potentially compromise stability. In the foaming ability assessment of the prepared soaps, the foam height of S1 closely resembled both S2 and S3 soaps.
Generally, the 2-PAM introduction to soap may affect the surfactant properties of the soap molecules. Surfactants reduce surface tension and enable the formation and stabilization of foam. If 2-PAM interacts with the surfactants, it could potentially enhance or inhibit the soap's ability to create and maintain foam. Higher concentrations of 2-PAM might lead to changes in the soap's viscosity, surface tension, or interfacial properties, all of which can impact foaming.
Table 3. Physicochemical analysis results of soaps
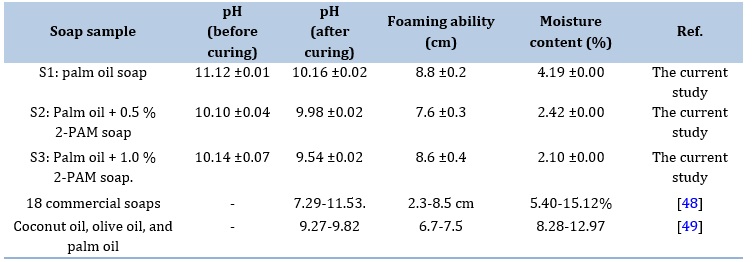
However, in this observation, the introduction of 2-PAM at low concentrations in the formulations of S2 and S3 did not unduly impact their foaming capabilities. Moisture content is a crucial quality parameter for solid soaps, impacting their shelf life and overall performance. In Table 2, the moisture content of S2 and S3 soaps is compared to S1. For S1 soap, the moisture content is 4.19 ± 0.00%, indicating a moderate level contributing to its texture and effectiveness. In contrast, S2 soap has a lower moisture content at 2.42 ± 0.00%, suggesting a drier composition. S3 soap has an even lower moisture content at 2.10 ± 0.00%, indicating a notably drier soap compared to both S1 and S2. These results show lower moisture content as compared to Malaysia palm- based soap properties described by Kuntom [50]. The 2-PAM inclusion in the soap formulation led to a reduction in moisture content, suggesting that the presence of 2-PAM reduced the soap's polarity, diminishing its ability to retain water molecules. Generally, solid soap is expected to have a low moisture content to ensure durability, maintain its shape and integrity for longer-lasting use, and prevent bacterial growth. According to literature, the maximum permissible moisture content in soap is 15 % [51].
If the moisture content is too low, the soap may become brittle and less effective, while if it is too high, the soap may become soft, prone to melting, and susceptible to bacterial growth. From the conducted physicochemical assessments, the incorporation of different concentrations of 2-PAM into the soap formulation does not compromise the intended physicochemical properties of the soaps. These findings underscore the exciting potential for utilizing these enhanced soaps as reliable decontamination agent.
Conclusion
In conclusion, proactive efforts have been made towards the development of a decontamination agent to address the challenges posed by organophosphates. The successful synthesis and comprehensive physicochemical characterization of 2-PAM plant-based soap, intended for degrading organophosphates, have been achieved. The FTIR analysis demonstrates the effective incorporation of 2-PAM into soaps S2 and S3, indicated by distinct bands at wavenumbers 3016 cm-1 to 3015 cm-1, 1736 cm-1 to 1735 cm-1, 1368 cm-1 to 1364 cm-1, and 1217 cm-1 to 1215 cm-1.
Beyond their chemical properties, these soaps exhibit favorable attributes, including a pH range of 10.16, 9.98 and 9.54 for S1, S2 and S3 respectively, a lathering capacity of 7.6 cm to 8.8 cm, and moisture content percentages of 4.19% for S1, 2.42% for S2, and 2.1% for S3.
While the study provides significant data for further research into their efficacy in organophosphate decontamination, several potential areas for improvement and limitations should be acknowledged. In this study, the investigation primarily focuses on commercially used oximes such as 2-PAM, prompting a suggestion for exploring a broader range of oximes and diversifying the range of organophosphorus compounds, given the variations in structure and toxicity among OPs. In addition, assessing the long-term stability of oxime-incorporated soaps and conducting toxicology studies would contribute valuable insights. Addressing these aspects will refine the study and advance the practical implementation of oxime-incorporated soaps for organophosphate decontamination.
Acknowledgements
This work was supported by the National Defence University of Malaysia Short Term Grant, UPNM/2022/GPJP/SG/10 and Research Centre for Chemical Defense for the facilities.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Orcid
Nur Shazwani Binti Abdul Latif : 0009-0000-5890-6313
Wan Md Zin Bin Wan Yunus : 0000-0003-0057-3784
Hafizah Binti Ariff : 0009-0003-2574-5332
Rauda Binti A. Mohamed : 0000-0001-7269-4505
Nurnadia Binti Andenan : 0000-0002-1811-1214
Khoirul Solehah Binti Abdul Rahim : 0000-0001-8189-2001
Noor Azilah Binti Mohd Kasim : 0000-0001-7034-304X
Ong Keat Khim : 0000-0002-6549-6540
Alinda Binti Samsuri : 0000-0002-4127-5161
HOW TO CITE THIS ARTICLE
Nur Shazwani Binti Abdul Latif*, Wan Md Zin Bin Wan Yunus, Hafizah Binti Ariff, Rauda Binti A. Mohamed, Nurnadia Binti Andenan, Khoirul Solehah Binti Abdul Rahim, Noor Azilah Binti Mohd Kasim, Keat Khim Ong, Alinda Binti Samsuri. Synthesis and Physicochemical Characterization of 2-Pyridine Aldoxime Methiodide (2-PAM) Incorporated Plant Based Solid Soap for an Organophosphate Decontaminant. Adv. J. Chem. A, 2024, 7(3), 278-288.
DOI: 10.48309/AJCA.2024.429332.1464