Document Type : Original Research Article
Authors
1 Department of Chemistry, University of Sistan and Baluchestan, Zahedan, Iran
2 Department of Chemical Engineering, Faculty of Engineering, University of Sistan and Baluchestan, Zahedan, Iran
3 Department of Chemical Engineering, Faculty of Engineering, Kermanshah University of Technology, Kermanshah, Iran
Abstract
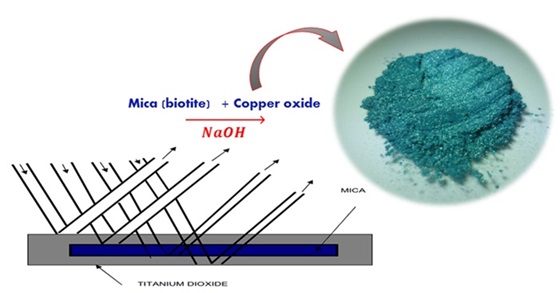
Pearlescent pigments encompass a diverse range known for their ability to create an aesthetically pleasing optical appearance due to the facile alignment of numerous platelet-like particles. Consequently, they serve as an ideal choice for imparting robust, lustrous color effects in powder coatings. The synthesis of green pearlescent pigments was conducted in this study, utilizing mica (biotite) and mica-titania flakes coated with a copper oxide thin film. The process entailed the uniform formation of copper (II) chloride through the addition of sodium or potassium hydroxide in the existence of titania, mica, mica-alumina, ammonium molybdate, or mica-titania flakes. Biotite, denoted by the formula K(Mg/Fe)3(A1Si3O10)(OH)2, was selected as the supporting mica. The synthesized pigments were characterized using X-ray diffractometry (XRD) and X-ray fluorescence (XRF) techniques. Evaluations were conducted using polypropylene extrusion with the incorporated pigments, revealing acceptable chemical resistance and distinctive pearl luster effects. The XRD pattern of the Cu/mica pigment exhibited the presence of copper hydroxide covering the mica surface, confirming the formation of the green pearlescent pigments.
Graphical Abstract
Keywords
Main Subjects
Introduction
Pearlescent pigments can display pearl shine when covered by a metallic oxide film due to angle-dependent optical effects by various refractive indices [1,2]. Due to the eye-catching effect of these pigments, they are widely used in industrial decorative and car painting [3,4]. Aquatic and organic environmental contamination due to various pollutants, such as heavy metals [5-7], aromatic compounds [8], pesticides [9], dyes [10,11], etc. [12,13] is consistently recognized as a notable concern for human health [14-16]. Pearlescent pigments are innocuous to human health (non-toxic) and eco-friendly compared to inorganic pigments [17]. Therefore, they are extensively used in various fields, such as cosmetics, children's toys, and food packaging [18]. Moreover, some pearlescent pigments have unique antimicrobial characteristics [19,20].
The photocatalytic oxidation property of these pigments led to the degradation of volatile organic compounds (the source of indoor air pollution) like acetaldehyde. Hence, pearlescent pigments could be applied as an indoor air purification system [21]. In addition, pearlescent pigments are used as a novel solar reflective building coating. They have high near-infrared (NIR) reflectance of about 85%. Application of these functional pigments mitigates the urban heat island (UHI) effect and sharply diminishes the cooling consumed energy and the related CO2 emissions [22]. These pigments can be suitable for creating unique color effects for pharmaceutical and industrial purposes. Commonly, the pearlescent pigments have significant stability in basic or acidic conditions and can also tolerate up to 800 °C [23]. These materials demonstrate exceptional resistance to weathering, sunlight, and heat, making them highly sought-after for use as coloring agents in applications where high temperature and paint resistance are crucial, such as porcelain enamel [24]. Pearlescent pigments possess unique properties that set them apart from other types of pigments. The characteristics encompassed in this category consist of electrical conductivity, susceptibility to laser reflection, and magnetic properties [25]. In contrast to the other pigments, in which scattering and absorption of visible light is the principle of their function, pearlescent pigments commonly consist of single crystals or multilayer structures and each layer may have diverse refractive indexes [26,27]. When these flakes are scattered in a translucent environment with a lower refractive index, they display an optical phenomenon that closely resembles natural pearls and shells. The interaction between the flakes and light in atmospheric conditions results in reflected and transmitted light, creating a unique visual effect [28]. According to the principles of thin-layer optics, the wavelength of light that is amplified through interference is directly influenced by two key factors such as the thickness of the optical layer and its refractive index. These critical components play a crucial role in determining the specific wavelength that will be enhanced through interference within an optical solid [29]. Pearlescent pigments based on mica or mica-titania can be synthesized via coating TiO2 or other metal oxides on thin layers of mica; these pigments are usually noticeable due to their acceptable chemical stability [30]. Several types of mica exist, such as sericite, phlogopite, biotite, muscovite, vermiculite, and lepidolite, which are various in chemical composition [31]. From the specific varieties of mica, muscovite is commonly used as a base for the colored pigments preparation. Mica-titania pigments are synthesized through the usage of various strategies. Lie et al. produced high glossiness silver pearlescent pigment by chemical vapor deposition (CVD) method in a fluidized bed reactor. The color of the pearlescent pigment was controlled by TiO2 concentration which effects on the deposited film thickness over the particle surface [32]. Zhu et al. used the liquid phase deposition to prepare of Fe2O3/flake α-Al2O3 pearlescent pigments. They showed that pH has the highest effect on the coating ratio [33]. The technique of heterogeneous nucleation in solutions is widely employed. This method includes the homogeneous hydrolysis and titration techniques as a substitute. Gao et al. prepared TiO2-coated mica-titania pigments using hydrolysis of TiCl4 ethanolic solution in water. Synthesized pigments showed a good photostability under ultraviolet light [34]. Xiaojuan et al. evaluated the effect of pH on the final properties of mica/Fe3O4 pearlescent pigments synthesized using co-precipitation method.
The obtained results revealed that in pH value equal to the co-precipitation pH of Fe3+ and Fe2+, pigments have a significant shine [35]. Furthermore, this technique has the potential to enhance the bonding between the metal oxide and the supporting surface. This study aimed to introduce an investigated and efficient method to prepare the green pearlescent pigments based on mica, titania, mica-titania, mica-alumina, and ammonium molybdate flakes covered with a thin film of copper oxide with usage the homogeneous precipitation of CuCl2 with sodium and potassium hydroxide.
Experimental
Materials and instrumentation
All reagents were obtained from Merck. In this work, the selected mica (biotite) was obtained from West Micronized Company and characterized by X-ray diffractomety. The CuKα irradiation was utilized to measure the powder X-ray diffraction patterns using the D8, Advance, Bruker, and diffractometer. X-ray fluorescence (XRF) analyses were carried out using spectrometer (Philips - PW2404).
Pearlescent pigments preparation
The first method: 1 g of titania/ mica-titania/ Mica/ ammonium molybdate was mixed with NaOH (6 ml - 1 M) and stirred to achieve the suspension state. 3 ml of CuCl2 dissolved in water was added to the mentioned suspension and mixed at ambient temperature for 15 min. The pigment powders with a pearlescent luster were obtained by filtering the resulting colored suspension, rinsing it with cold water, and allowing it to dry in the air.
The second method: A suspension was formed by stirring 1 g of Mica with 6 ml of 1 M KOH solution. The mixture was then heated and stirred at 60 °C for 1 hour. Next, 3 mL of 1 M CuCl2 aqueous solution was added to the obtained mixture and stirred under 60 °C for 1 hour. Once the reaction was complete, the mixture was cooled to ambient temperature, filtered, and washed twice with cold water. Finally, the pigment powders were obtained after drying in air.
The third method: The combination of 1 g of mica-alumina and 0.36 g of NaOH was subjected to heat in an electric furnace until the mixture melted. Subsequently, 0.48 g of transition metal salt was introduced into the molten mixture and heated at 400 °C for 10 minutes. After cooling to ambient temperature, the resulting mixture was dissolved in 100 ml of water and filtered. The colored pigment obtained was washed with cold water and air-dried to obtain the pigment powders.
Pearl pigments extrusion
A specific quantity of polypropylene (3 g) underwent heating in an oven at a temperature of 180 °C for 12 hours. Subsequently, 10% (w/w) copper pearlescent pigment (prepared using method 1) was introduced and physically blended. The resulting blend was then extruded through the extrusion apparatus, resulting in the formation of a lustrous green wire.
Results and discussion
The selected mica was biotite, which was characterized via X-ray diffraction analysis. The copper oxide-coated minuscule mica plates were responsible for the generation of color interference. The pigments were synthesized through three different methods, all of which yielded satisfactory results. A summary of these outcomes can be found in Table 1. It should be noted that each of the tests presented in Table 1 has been repeated at least 3 times and the Yield% was listed as an arithmetic average.
As indicated in Table 1, pigments produced via the first and the second methods had the same quantity and quality, approximately. The first method was suggested as the proper approach for cost-effective production of metallic pearl pigments, with mica being identified as the most suitable support for pearlescent pigments in the aforementioned methods. Due to the removal of water from hydrated compounds and the subsequent transformation of these compounds into oxides [36], the pearlescent pigments produced by the third method had a black color. Figure 1 demonstrates how the pearl pigments align parallel to the substrate surface in a transparent medium. This alignment allows for the incident light to be consistently reflected off each pigment plate, resulting in the desired and proper pearl luster effects.
Table 1. Various techniques for producing pearlescent pigments with different yields
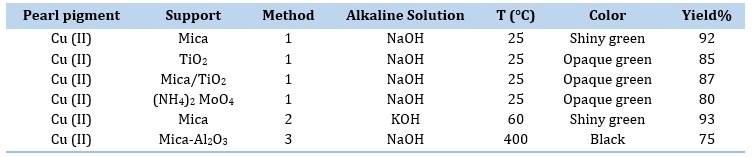
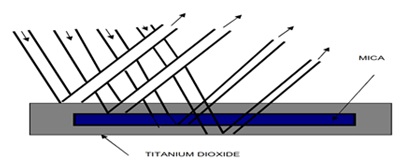
Figure 1. Regular multiple reflections.
The micrograph displayed in Figure 2 serves as a representative example of the pearl pigments. This Figure illustrates that the support flakes were covered with a sleek and lustrous colored coating of transition metal oxides or hydroxides on mica.
Shiny green polymer wires were produced through the polypropylene extrusion with copper/mica pearlescent pigments (Figure 3).
X-ray diffraction analysis
The XRD patterns of the utilized particles as substrates in this investigation are depicted in Figure 4. It was disclosed that the chosen mica had existed in the biotite structure.
Figure 5 displays the XRD patterns of copper pearlescent pigment. The pearlescent pigments exhibited crystalline properties, and the XRD patterns of Cu/mica pigments indicated that the green pearlescent pigments contained metal oxides and were deposited onto the mica and titania surface.
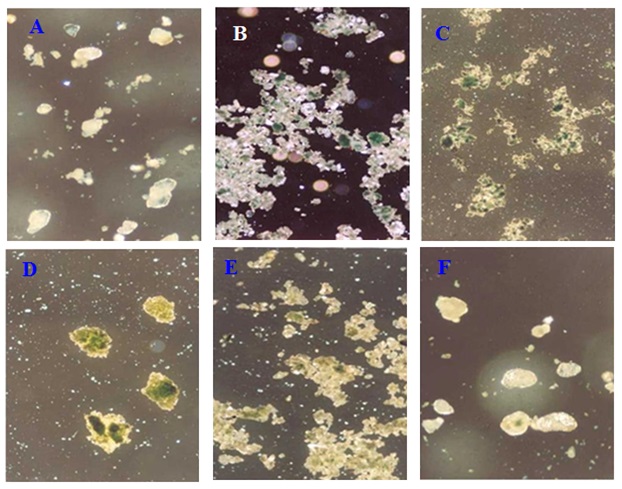
Figure 2. Representative micrograph of chosen pearl pigment (A) Cu/mica pearl pigment prepared by the first method (magnification*100), (B) Cu/mica pearl pigment prepared by the first method (magnification*40), (C) Cu /mica-TiO2 pearl pigment prepared by the first method (magnification*40), (D) Cu /TiO2 pearl pigment prepared by the first method (magnification*100), (E) Cu /mica pearl pigment prepared by second method (magnification*40), and (F) Cu /mica pearl pigment prepared by the second method (magnification*100).

Figure 3. The polypropylene extrusion with copper pigment.
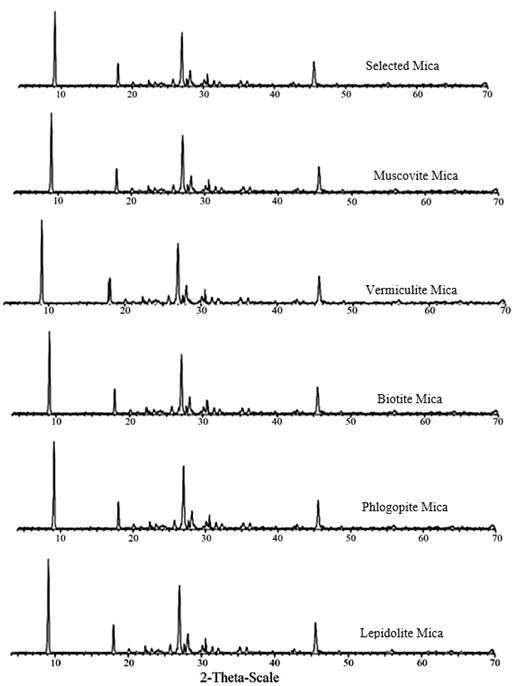
Figure 4. Comparison between the XRD pattern of the chosen mica and various types of mica XRD.
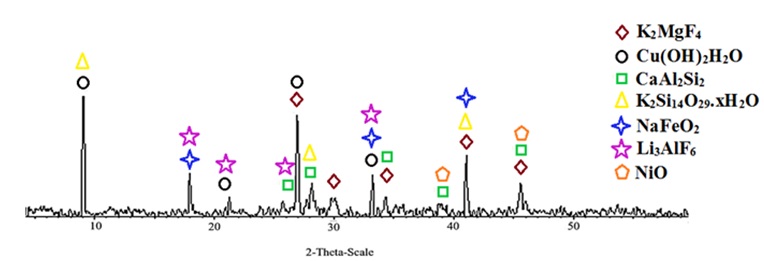
Figure 5. XRD pattern of Cu/mica pigment in the NaOH presence.
Table 2. XRF analysis data versus components percentage for Cu/mica-TiO2 pearl pigments
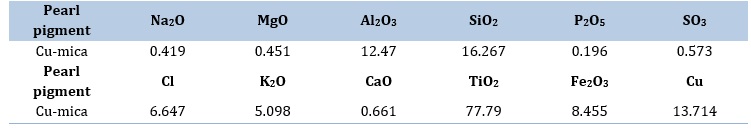
Figure 5 also revealed that the actual phases of Cu/mica pigment were K2MgF4 (Tetragonal), Cu(OH)2 H2O (Triclinic), CaAl2Si2 (Hexagonal), K2Si14O29.xH2O, NaFeO2 (Rhombohedral), Li3AlF6 (Cubic), and NiO (Rhombohedral). According to Figure 5, the x-ray scattering on Cu/mica pigment exhibited the diffraction pattern characteristic of the K2MgF4 (Tetragonal) type orthorhombic structure in 27, 30, 34.5, 41, and 45.5 for 2-theta (degrees). The position of the peaks obtained at (9, 21, 27, and 33), (26, 28, 34.5, 39, and 45.5), (9, 27, 28, and 41), (18, 33, and 41), (18, 21, 26, 33, and 41) and (39 and 45.5) is considered to contribute to the phase of Cu(OH)2 H2O (Triclinic), CaAl2Si2 (Hexagonal), K2Si14O29.xH2O, NaFeO2 (Rhombohedral), Li3AlF6 (Cubic), and NiO (Rhombohedral), respectively (Figure 5).
XRF analysis of copper pearlescent pigment
The XRF technique confirms that the biotite chemical composition is K(Mg/Fe)3(A1Si3O10)(OH)2. Table 2 presents the XRF analysis data of Cu/mica pearl pigment, which reveals the presence of Cl, Si, Cu, K, Fe, Ca, Al, and Mg elements in the mica.
Conclusion
To summarize, a variety of metallic pearl pigments have been synthesized and analyzed using XRD and XRF techniques. The pigments exhibit excellent chemical resistance and produce a captivating pearl luster. Various colors were obtained with coating titania, mica/titania or minuscule mica flakes. The formula of the selected mica, biotite, was K(Mg/Fe)3(A1Si3O10)(OH)2, which was confirmed by the XRD technique. The XRF data revealed that mica contains Mg, Si, Cu, Ca, Cl, Fe, Al, and K elements. XRD pattern of Cu/mica pigment shows that the green pearlescent pigment contains copper hydroxide that covers the surface of mica. The first and the second methods yield a similar quantity and quality of the product. As a result, method 1 is highly recommended for the production of metallic pearl pigments due to its cost-effectiveness. In addition, mica proves to be the proper choice as a support material for creating pearlescent pigments using these techniques.
Orcid
Mozhgan Zakeri : 0000-0001-9988-5176
Hamid Moghadam : 0000-0001-5419-6334
Mohsen Samimi : 0000-0003-3098-7283
HOW TO CITE THIS ARTICLE
Marzieh Cheraghipoor, Mozhgan Zakeri, Hamid Moghadam, Mohsen Samimi* A Feasibility Study for the Preparation of Green Copper-Colored Mica Pearlescent Pigments. Adv. J. Chem. A, 2024, 7(3), 338-346.
DOI: 10.48309/AJCA.2024.436923.1480