Document Type : Original Research Article
Authors
1 Department of Chemical Engineering, Mahshahr Branch, Islamic Azad University, Mahshahr, Iran
2 Department of Chemistry, Mahshahr Branch, Islamic Azad University, Mahshahr, Iran
3 Department of Chemistry, Shoushtar Branch, Islamic Azad University, Shoushtar, Iran
Abstract
A magnetically separable catalyst n-butylpyridinium tetrachloroferrate ([C4py]FeCl4) was designed for the removal of trace olefins from aromatic hydrocarbons. The magnetic ionic liquid was prepared via a facile synthetic route and well characterized by FTIR, TGA/DTG, DTA, UV–Visible, and Raman techniques. Then, the effects of different reaction parameters such as molar ratio, reaction temperature, reaction time, and the reusability of the ionicliquid were investigated. It was found that the ionic liquid ([C4py] FeCl4) with 5 wt% of the catalyst at 70 °C for 12 min., exhibited a better performance for olefin removal.The magnetic IL was easily removed from solution using an external magnet, allowing efficiently recovery and without work-up or purification processes.
Graphical Abstract
Keywords
Introduction
Alkenes streams produced from naphtha reforming or thermal cracking are commonly found in aromatic hydrocarbon.Also, olefins are by-products of petrochemical processes such as isomerization and trans alkylation [1-2]. These trace olefins have high chemical activities to form resins which will affect the quality of aromatics, and can cause some adverse effects on subsequent processes. The most common practice for removing olefin contaminants involves clay treating process and catalytic hydrogenation treatment. However, both approaches have some drawbacks; such as high temperature, high pressure, simple inactivation, short life cycle, and severe environmental contamination [3]. Recently, a varity of new green acid catalysts such as ionic liquids [4-5], modified zeolites [6-7] and solid superacid catalysts [8-10] have been developed to overcome the problems that these classical approaches do not solve.
Sustainable development as well as the circular economy and environmental issues are at the leading public and government concern. The green chemistry field aims to provide environmentally benign products from sustainable resources that use processes not to harm people or the environment and help to solve key societal problems [11]. Catalysis, including homogeneous, heterogeneous, and bio-catalysis is the key chemical processes to achieve the objectives of sustainable green chemistry [12-20]. The use of a number of room- temperature ionic liquids (RTILs) as catalysts in organic reactions brings undeniablebenefits considering both the principles of green chemistry and the economic point of view [21-22]. ILs containing magnetic metals in their structure are known as magnetic ionic liquids (MILs) and they present unique physicochemical properties. Incorporating various types of magnetic metal anions in ILs gives a powerful response to magnetic field and in turn has the advantage of catalyst recyclability [23-32].
Considering what stated above and in continuation of our systematic research on the design and synthesis of paramagnetic catalysts [33-39], we have prepared and characterized n-butyl pyridinium tetrachloroferrate [C4py] FeCl4as a magnetically recyclable catalyst for removal of trace olefins from aromatics. To the best of our knowledge, this is the first report of using a task specific metal-containing ionic liquid in this type of reactions.
Experimental
Materials and Methods
The aromatic hydrocarbons were obtained from PX combinationunits at Bandar Emam Petrochemical Company (Iran) with a Bromine Index (BI) of195 mg Br/(100 g) and according to GC analysis, the major component was 92.47 wt% of benzene, and 2.55 wt% of toluene. All other compounds were analytically pure and commercially obtained from Sigma-Aldrich (USA) and Merck (Germany) companies and were used as received without any further purification. Fourier transform infrared (FT-IR) spectra of the catalyst wererecorded on a Bomem MB-1998 in KBr disks.Thermogravimetric analysis (TGA) was performed with a heatingrate of 10 °C/min in nitrogen. Raman measurements were performed on an Almega ThermoNicolet Dispersive Raman spectrometer at a wavelength of 480 nmof a Nd:YLF laser.
Catalyst Preparation
A typical procedure for the preparation of n-butyl pyridinium tetrachloroferrate [C4py]FeCl4was asfollows [40]. A mixture of 1-Chlorobutane (2.0 mmol) and pyridine (1.0 mmol) was heated at 78 °C for 72 h under vigorous stirring. The obtained [C4py]Cl was washed three times with hexane and dried in vacuum. Then, [C4py]Cl (1.0 mmol) and FeCl3 (1.0 mmol) in 10 mL of dry methanol were stirred at room temperature for 24 h. The product was filtered, washed with diethyl ether (10 mL), and dried in a vacuum oven at 60 °C (Scheme 1).
Scheme 1. Synthesis of[C4py]FeCl4
Catalytic Activity Evaluation
The quality of aromatic products was quantified by the BI, that is defined as the number of milligrams of bromine consumed by 100 g of hydrocarbon sample. Therefore, the value of the BI was an indication of the relative amount of olefins. In current study, the catalyst activity was defined as the conversion of olefins: X = [(BIo-BIi)/BIo]×100%, BIo and BIi were the raw materials and products bromine index, respectively.
Results and discussion
Catalysts Characterization
TheFT-IR spectrum of [C4py] Cl shows characteristicstretching frequencies at 3131, 3083,2962, 2931, 2875, and 1635 cm−1 due to stretching vibrations of C-H (aromatic rings and linker moiety), and C=N respectively(Figure 1b). The absorption band at 3416 cm−1 is assigned to stretching vibrations of adsorbed water molecules on the surface of [C4py] FeCl4. The peak observed at approximately 1450 cm−1 is attributed to pyridine interacting with Lewis-acid sites; while a band near 1490 cm−1 is considered as combined interaction by both Bronsted and Lewis-acids. The band observed at 1173 cm−1 is related to the stretching vibration of C-N. The peaks at 775 and 690 cm−1 are assigned to the out-of-plane bending vibrations of =C–H and C=C groups, respectively. These absorption bands also appeared in FT-IR spectrum of [C4py] FeCl4, which confirmed the presence of [C4py] in [C4py] FeCl4 (Figure 1c). Additionally, the absorption associated to Fe-Cl bond vibration in FeCl3 and [C4py] FeCl4 is observed at approximately 420 cm−1, which was not observed in [C4py] Cl.
Figure 1. FT-IR spectrum of FeCl3 (a), [C4py] Cl (b), and [C4py] FeCl4 (c)
The thermal stability of the catalyst was investigated by TGA/DTG and DTA at various temperatures ranging from 25–625 °C (Figure 2). The weight loss (15%) from the [C4py] FeCl4 catalyst (25 °C to 125 °C) is due to the removal of physically adsorbed water and organic compounds, which were applied in the catalyst preparing. In the range of 125–350 °C, which is accompanied by a mass loss of 12% corresponds to the elimination of the linker organic moiety from ionic liquid. After 350 °C, weight loss rate of [C4py] FeCl4 is accelerated significantly; the tiptop temperature is 400 °C. This weight loss (48%) belonged to the thermal decomposition of the organic cation of the catalyst. In the DTA curve (Figure 2b), in addition endothermic peaks associated with different mass losses observed in TG, exothermal signal assigned to the loss of main n-butylpyridinium, was observed with maximum at 400 °C.
Figure 2. TG-DTG analysis for [C4py]FeCl4 (a) DTA measurement for [C4py]FeCl4 (b)
The Raman spectrum of [C4py] FeCl4 is shown in Figure. 3. In the range of 50–500 cm-1, two peaks at ~112 cm-1, and ~332 cm-1 are corresponding to the symmetric Fe–Cl bond stretch vibrations. The peak observed at 475 cm−1 does not assign with a known FeCl4− feature, which may be due to the [C4py] cation. Thus, it can be confirmed that the catalyst contain FeCl4− anions [41].
The ultraviolet and visible absorption spectrum of [C4py] FeCl4 is shown in Figure 4. The characteristic peaks of [C4py] FeCl4 have appeared in 218, 257, and 362 nm in accordance with the literature. These results confirmthe presenceof particle [FeCl4–] in the anionic form in the composition of magnetic ionic liquids [42].
Figure 3. Raman spectrum of [C4py]FeCl4
Figure 4. Ultraviolet and visible absorption spectrum of the synthetic [C4py]FeCl4
Optimization of the Reaction Conditions
After preparation and characterization of [C4py] FeCl4catalyst, its catalytic activity wasinvestigated on removing trace olefins from aromatics. In order to obtain the optimum conditions, initially, the effect of reaction temperature was investigated by carrying out the reaction at various temperatures for 12 min and using a catalyst amount of 5% (wt/wt) (catalyst to oil ratio). The best result was obtained at 70 °C, and the olefins conversion could reach 98%. Increasing in reaction temperature leads to a decrease of current efficiency to 92% caused by the emission of the volatile precursors from the reaction mixture (Figure 5). To determine the catalyst amount, the reaction mixture was performed in the presence of various amounts of [C4py]FeCl4 at 70 °C for 12 min. The results showed when the mass ratio of IL to oil increased from 2 to 5%, the olefin conversion increased rapidly and the use of larger amounts of the catalyst (6% wt/wt) did not improve the conversion (Figure 6). The effect of reaction time on olefin conversion was also investigated in the presence of5 wt% of the catalyst at 70 °C by varying the reaction time from 2 to 20 min. According to the obtained results, the conversion increased as the reaction time was prolonged from 2 to 12 min., and no improvement was detected in the conversion after 12 min (Figure 7).
Figure 5. Effect of reaction temperature on olefin conversion
Figure 6. Effect of catalyst amount on olefin conversion
Figure 7. Effect of reaction time on olefin conversion
Taking into consideration the above results, the following mechanism for olefins removal, which is one kind of alkylation reactions is proposed. Initially, olefin molecule is activated using the MIL catalyst to provide carbonium ion. After that, aromatic compound attacks the activated olefin, and gives alkylbenzenium ion. Subsequently, through removing one proton from alkylbenzenium ion, alkylated aromatic is prepared.
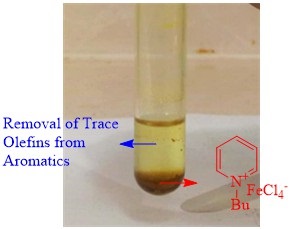
It is important to note that the magnetic property of [C4py] FeCl4facilitates its efficient recovery from the reaction medium. For this purpose, the reusability of [C4py] FeCl4was investigated in the model reaction under the optimized reaction conditions for five consecutive cycles. After the completion of the reaction, the catalyst was separated using an external magnet and washed with diethyl ether (10 mL) and dried in a vacuum oven at 60 °C. The results indicated that [C4py]FeCl4can be reused up to 5 times with moderate loss of the catalytic activity and theconversion difference between the first and 5th runs is only 9% (Figure 8). Figure 9 displays the magnetic separation ability of the catalyst using magnetic field force, indicating that the metal-containing ionic liquid has good magnetic property.
Figure 8. Reusability of the catalyst
Figure 9. [C4py]FeCl4 deposited at the bottom of a glass tube and attracted to a NbFeB magnet
Conclusion
In summary, a green and reusable organocatalyst n-butylpyridinium tetrachloro-ferrate is prepared and characterized. The catalytic activity of the MIL is studied for removal of trace olefins from aromatics. The catalyst [C4py]FeCl4 promotes the reaction rate and also simplifies the work-up procedure. Some attractive features of the protocol are excellent yields, short reaction times, easy work-up, high catalytic activity, eco-friendly benign and recyclability of the catalyst. The catalyst can be used several times without a substantial reduction in its catalytic activity.
Acknowledgement
We are scincerely grateful to the Mahshahr Branch, Islamic Azad University and Bandar Imam Petrochemical Company for support of this work. We are also grateful to Dr. M. Fallah-Mehrjardi for his helpful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.