Document Type : Original Research Article
Authors
1 Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
2 Department of Chemistry, Federal University Gashua, Yobe State, Nigeria
Abstract
The use of plant products as medicines could be traced as far back as the beginning of human civilization. The medicinal plant “Strychnos innocua (a Loganiaceae plant)” is a deciduous shrub growing 2-12 m tall. This study aimed at using GC-MS to determine the chemical composition and antifungal activity of a root bark methanol extract against Candida krusei. The plant sample was collected and identified at Ahmadu Bello University, Zaria. The maceration extraction method was used to obtain the methanol root bark extract. The chromatogram from the GC-MS analysis revealed sixty-four (64) peaks corresponding to fifty-five phytoconstituents from various organic groups. Among the significant compounds discovered are 9,12-Octadecadienoic acid (5.48%), Benzofuran, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-, trans- (6.72%), Tetradecanoic acid, 12-methyl-, methyl ester (6.87%), Ethyl Oleate (8.29%), 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester (9.10%), Linoleic acid ethyl ester (10.31%) with 6-Octadecenoic acid, methyl ester and peak area of 11.42% being considered the highest peak area. In contrast, Bicyclo[13.1.0]hexadecan-2-one with a peak of 0.03% is considered the lowest peak area. It can be concluded that the phytocompounds discovered in the root bark methanol extract of Strychnos innocua could be valuable in treating some human ailments.
Graphical Abstract
Keywords
Introduction
Herbs have been used for pharmacological therapy of disease for a long time. Herbs were extensively used in traditional folk healing methods throughout the world [1]. Since the dawn of civilization, plant-based medications have been used as medicines [2]. Researchers studying medicinal plants have looked at various low-cost targets such as prototypic drug discovery and therapeutic compounds development [3].
Secondary metabolites such as flavonoids, alkaloids, triterpenes, glycosides, saponins, tannins, carbohydrates, and essential oils, among others, contain a variety of complex chemical substances that contribute to medicinal plants' therapeutic properties [4, 5]. Various antimicrobial activity investigations of herbal plant extract have shown potential antibacterial efficacy against bacterial and fungal pathogens [25]
Strychnos innocua is a member of the Loganiaceae family. The plant is a deciduous shrub with numerous branches that grows between 2 m and 12 m tall and has a smooth, green, or yellow-white powdery bark. However, some plant specimens can grow to be 18 m tall. The 7–40 cm diameter straight trunk is commonly branched from low down [6]. The root decoction is taken as a remedy for gonorrhea, and the fresh roots are used to treat snakebite [6, 7].
Strychnos innocua fruits have been studied for their nutritional, antinutritional, and chemical composition [8-10]. This study aimed to use GC-MS to determine a root bark methanol extract's chemical composition and antifungal activity against Candida krusei.
Figure 1. Strychnos innocua, showing the leaves and branches
Methods
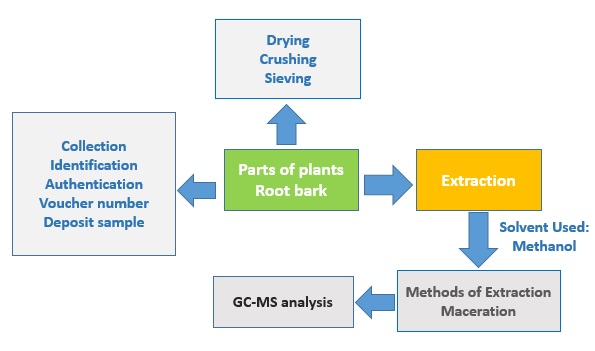
Collection and Identification
The plant materials were collected from the wild, Soba Local Government Area, Kaduna State, Nigeria. It was then authenticated by Mallam Namadi Sunusi of the herbarium section of the Department of Biological Sciences, Ahmadu Bello University, Zaria – Nigeria. A voucher specimen number (V/N – 01884).
Preparation and extraction of plant material
Then, the root bark was rinsed, air-dried for 28 days, and crushed to a coarse powder. The pulverize plant sample (2000g) was separately macerated successively in n-hexane solvent, ethyl acetate solvent, and methanol solvent according to gradient polarity. The maceration technique involved soaking the pulverized plant materials in an aspirator first with n-hexane (polarity = 0.009) and standing at room temperature for 3 days with frequent agitation. After exhaustive extraction with n-hexane, the procedure was repeated for ethyl acetate (polarity = 0.228) and methanol (polarity = 0.762).
GC-MS Analysis
GC-MS analysis of S. innocua methanol root bark extract was performed using GC 7890B, MSD 5977A, Agilent Tech.
The sample was dissolved in ethanol and votex mixed for 1 min followed by centrifuging at 3000 rpm for 10 min. The GC was injected with supernatant (1 µL) and ran for 30 min.
The volatile compounds contained in the sample were analyzed by the GC/MS on an Agilent 7890B gas chromatography (GC) directly coupled to the mass spectrometer system (MS) of an Agilent 5977 A. The ionization voltage and ion source were set at 70 eV and 230 °C, respectively. The oven temperature of the GC was programmed from 80 °C, increased at 15 °C/min to 200 °C, then 5 °C/min to 280 °C, finally end with a 5 min isothermal at 280 °C. Helium was the carrier gas used at 1 mL/min flow rate.
Antifungal Activity
The antifungal was tested against Candida krusei and Aspergillus nigre. The growth medium was Sabouraud dextrose agar (SDA), and the screening method was the agar well diffusion method. In addition, 0.1 mL of the fungi's standard inoculum was seeded into freshly produced SDA. A sterile cork borer with a diameter of 6 mm was then used to punch a well in the center of the medium. After that, 0.1 mL of the appropriate extract concentration solution was added to the well. The SDA plates were incubated at 30 °C for 7 days. The growth inhibition zone was then seen on the media plates. The zone was measured with a clear ruler, and the result was recorded in millimeters (mm). Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) were determined using broth dilution [26, 27].
Results and Discussion
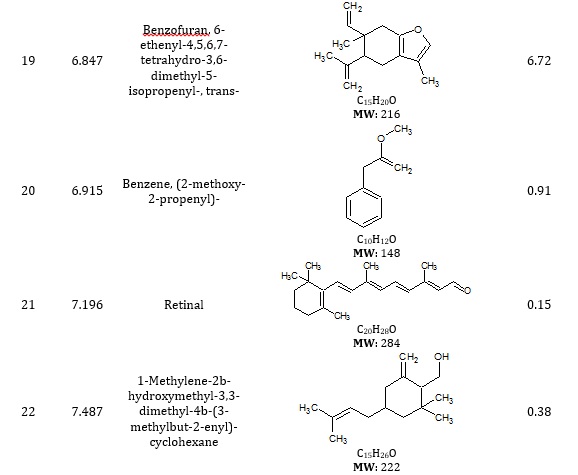
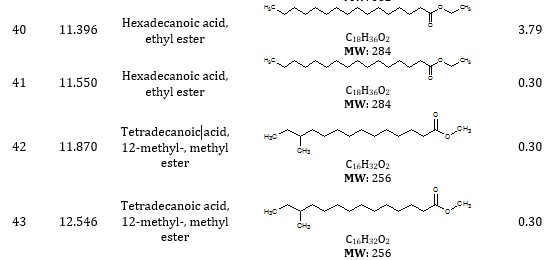
The combined gas chromatography and mass spectrometry (GC-MS) are used to identify distinct volatile compounds in a sample. The GC-MS chromatogram revealed the presence of fifty-four (54) peaks corresponding to forty-five (45) phytoconstituents from various organic groups. The chromatograms in Figure 1a and Figure 1b revealed the peaks based on RT and area %. The phytoconstituents identified in the extract of methanol root bark of Strychnos innocua are presented in Table 1.
Table 1. Phytoconstituents of the extract of methanol root bark of Strychnos innocua
Figure 2. The chromatogram of phytoconstituents in methanol root bark extract, 1-10 min (a), 10-27 min (b)
Table 2. Antifungal activity of the methanol root bark extracts of S. innocua
Table 2 shows the zone of inhibition (mm), MIC, and MFC of methanol extract from the root bark of S. innocua.
Silanediol dimethyl was found in citrus limon and papaya after an investigation of microbial concentration [11]. Ethylbenzene production for commercialization has been reported in industries using the benzene alkylation process [12]. One of the primary compounds found in Pseudomonas aeruginosa with antimicrobial activity is oxime-, methoxy-phenyl [13]. Hexadec-7-enal has been identified among compounds that elicited electroantennogram responses from white-tailed bumblebees [14]. Ethyl oleate is a fatty acid ester and safe for oral ingestion [15] and is used as a solvent for pharmaceutical drug preparations [16]. Linoleic acid and ethyl ester have been reported to effectively treat infantile neuroaxonal dystrophyl [17]. 9,12-octadecadienoic acid (Z, Z) was also discovered in Gossypium seeds and had antimicrobial activity [18]. 6-octadecenoic acid, methyl ester was found in Staphylococcus and is highly effective in suppressing the growth of Aspergillus terreus [19]. Hexadecanoic acid methyl ester, known as palmitic acid methyl ester, was reported to contribute as paracrine perivascular relaxing factors to the regulation of vascular contraction [20]. Bicyclo[13.1.0]hexadecan-2-one was discovered in methanol extracts of Diadema setosum [21], Thaumatococcus danielli seed [22], and Catharanthus roseus leaf extract [23]. γ-Elemene, along with other compounds found in Tridax procumbens, has anticancer activity [24].
The antifungal activity of the methanol extract was recorded against C. krusei and A. nigre, which are of variable values. However, the zone of inhibition was presented in Table 2, which showed excellent activity against C. krusei, a nosocomial pathogen found immunocompromised with natural resistance fluconazole [28] and causative of Candidaemia infection. For C. krusei and A. nigre, the zone of inhibitory activity is 34.00 mm and 0.00 mm, respectively. There was no activity against A. nigre. The antifungal properties of this extract could be attributed to the synergistic therapeutic effects of the phytoconstituents shown in Table 1, which may have contributed to the antifungal activity against C. krusei [29]. The concentration of extract that inhibits C. krusei growth (MIC) is 5 mg/mL. In comparison, the concentration of extract that kills C. krusei (MFC) is 10 mg/mL, indicating the extract's potency against C. krusei.
Conclusions
GC-MS analysis of root bark methanol extract of Strychnos innocua showed a highly composite profile of phytoconstituents which include 6-Octadecenoic acid, methyl ester at the highest peak area (11.42%), and bicyclo[13.1.0]hexadecan-2-one at the lowest peak area (0.03%). Hence, it is concluded that the revealed phytocompounds in root bark methanol extract of Strychnos innocua might be highly valuable in medicinal usage to remedy some human ailments.
Acknowledgments
The authors are grateful to Mallam Samaila Mustapha for providing the plant. We are also thankful to Mallam Idris of Chemistry Laboratory, Yobe State University Damaturu, for his valuable technical assistance in GC-MS analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ahmed Jibrin Uttu : 0000-0002-4089-5529