Document Type : Original Research Article
Authors
1 Department of Chemistry, Kogi State College of Education, Ankpa, Kogi State, Nigeria
2 Department of Chemistry, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
Abstract
Vegetable-based natural esters serve the dual purpose of insulating and cooling electrical transformers as a natural alternative to conventional mineral oils. The factory-functionalized SiO2 nanoparticles were dispersed into purified cotton seed oil methyl ester to produce a nanofluid with excellent electrical and thermal properties. A dispersion method was used to prepare the nanofluids with 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8 wt. % nanoparticles into the ester at 0.1 wt. % increments. Scanning electron microscopy (SEM) coupled with electron dispersive X-ray (EDX) analysis was performed on the nanoparticles to determine their morphology and elemental composition. This study compares the results of testing samples of mineral oil, ester, and SiO2-based nanofluids before and after aging to determine their water content, flash point, pour point, dynamic viscosity, density, dielectric loss, permittivity, and breakdown strength. The results showed that CNF5 (Ester + 0.5 wt. % SiO2) performs better even after the samples have aged, owing to the addition of 0.8 wt. % SiO2 nanoparticles. CNF5 outperformed the other samples of nanofluid in terms of both thermal and dielectric properties. For CNF5, it was observed that the breakdown voltage, flash point, dynamic viscosity, tan delta, pour point, and water content decreased after ageing. It was also found that the cottonseed oil-based nanofluid improved the electrical and thermal properties more than mineral oil as nanoparticles were added to the samples.
Graphical Abstract
Keywords
Introduction
High demands have been placed on the dependability and performance of insulating materials used in the electric power system. These demands have resulted in the developing of future high-voltage networks and the smart grid [1]. The most significant component of an electric power network is a transformer, which changes the voltage and transports energy. Failure of a transformer could have disastrous consequences [2,3]. Most transformers in use today are either at the end of their design life or have already passed it; for this reason, the functional reliability of these existing units has received a lot of attention. According to available statistics on transformer failure, insulation issues reduce the typical service life of transformers to 17.8 years. This is less than half the projected lifespan of 35 to 40 years [4,5], and problems with dielectric insulation were the cause of 75 % of high-voltage transformer collapses. Transformer operational dependability and lifespan are primarily influenced by the characteristics and state of the insulating material [6]. Transformers are one of the key components of the electrical system that must be well-maintained and free from malfunctions. The transformer uses mineral oil as insulation and a cooling medium [7]. Since mineral oil is not biodegradable and has excellent dielectric properties but is highly stable at high temperatures, an ester-based fluid, which is ecologically friendly, has been introduced as an alternative. The fluid should have an extremely elevated flash point and fire point, indicating that it is stable enough at higher temperatures. A transformer typically operates at high temperatures, and there is also a danger of catching fire [8]. Higher breakdown voltages of mineral oil are stable enough to endure intense electrical stresses. On the other hand, it also functions across a wide temperature range and has superior thermal characteristics, such as high flash and pours points. Transformer oil is non-biodegradable and toxic. Its dielectric and thermal properties deteriorate due to the continuous use of the transformer. In addition to deterioration, crude oil being non-renewable may cause the extinction of mineral oil reserves shortly [9]. These features suggest a shift toward some acceptable substitutes, such as natural ester oils (vegetable oils), because of their benefits, such as biodegradability, lower caloric value, and enhanced values for flash point, thermal conductivity, and temperature stability [10]. Heat and electrical discharge in the transformer cause the insulation oil to deteriorate while it is in use. The cooling and insulating properties of insulation oil decrease with age. Therefore, oil quality needs to be maintained and regularly checked to ensure the transformer's operational reliability. Due to constant use, insulating oil produces dust and water particles as well as some acidic compounds and oxides, which reduce the breakdown strength of the oil [11]. Developing transformer oil with favorable dielectric and thermal characteristics is essential to meet the growing need for high voltage rates and the small size of transformers [12]. As a result, nanofluids have been produced to fulfill the aforementioned essential properties. Recently, transformer oil has been infused with ester-based nanoparticles to increase its breakdown strength, ageing, and insulating capabilities. Due to their small size (on the order of 20 nm) and low density, nanoparticles do not disperse in the presence of gravity [13]. Literature has shown that using nanoparticles and insulation oil can enhance electric strength, thermal conductivity, and efficient heat transmission [14]. By slowing down the streamer and streamer dispersion, nanoparticles serve as electron traps and help to increase the dielectric strength. Compared to base oil without nanoparticles, oil containing these nanoparticles has a more effective capacity for heat transfer. These improved characteristics result in a reduction in the size of high-voltage equipment [15]. Previous research has shown that the most effective metal oxide nanoparticle in displacing oil and improving its recovery is SiO2, with 2000 ppm concentration [16]. Transformer oil must be operated efficiently as it is a crucial electrical system component. In transformers, oil is utilized as an insulating medium that also aids in cooling and arc detection [17]. As a result of its advantageous properties, vegetable oil is utilized as an insulating medium in transformers, acting as an alternative to mineral oil [18]. To increase power transformer efficiency, it is necessary to control and monitor the characteristics of the insulating oil. Vegetable oil does lose some of its dielectric properties over time and no longer helps to keep the transformer cool. Therefore, certain nanoparticles are added to the ester-based fluid to enhance performance, insulation, cooling efficiency, thermal conductivity, breakdown strength, and age. The dielectric characteristics, heat transport, and thermal conductivity are all greatly improved by nanoparticles [19]. Similarly, previous research has found that compared to non-modified oil, the nanooil has better thermal and dielectric properties [20]. As the concentration of Boron Nitride nanoparticles increases, results show a significant improvement in the heat transfer process. Accelerated aging is used to forecast insulating material dependability during a given period of operation. The electrical, thermal, and mechanical loads that transformers are exposed to during operation should be included in an ideal ageing test. However, thermal stress predominates the ageing factors; hence, accelerated thermal ageing was employed in the simulated ageing test to induce the ageing mechanisms quickly. Similar experiments were conducted at 120 0C using wet coconut oil and wet and dry transformer oil. During various aging periods of 2, 4, and 7 weeks, changes in color, interfacial tension, acidity, breakdown voltage, and frequency dielectric spectroscopy were noted. In the aging process, coconut oil's acidity and conductivity levels increased. The fact that it had a higher breakdown strength than transformer oil, thus, suggested that it would be suitable for use as transformer liquid insulation [21]. Additionally, high dielectric permittivity CCTO (CaCu3Ti4O12) nanopowder was used to measure the electrical and physical parameters such as breakdown voltage, loss tangent, viscosity, interfacial tension, and flash point at room temperature following IS requirements. According to increases in particle volume fractions and temperature, it was found that adding nanopowder to pure and aged mineral oil produced better breakdown voltage and flash point results [22]. The breakdown strength of nanofluids under all test voltages was 20 % higher for transformer-based nanofluids than for base oils, according to the test results. In addition, the partial discharge resistance of the nanofluid was improved. More so, under accelerated thermal aging conditions for 6 days at 130 °C, TiO2 nanofluid demonstrated good anti-aging ability. Aged nanofluid had an AC breakdown voltage that was 1.19 times higher than that of aged base oil [23]
Data on the ageing behavior of ester-based oil is still relatively scarce, and researchers still face difficulties simulating the insulation system in transformers in the laboratory. A comparison of accelerated aged mineral oil and natural ester insulating oil is frequently used to gather information on the ageing behavior of natural ester-based insulating fluid since it significantly affects the thermal behavior of a mineral oil-based insulating fluid. In this study, several oils, including mineral oil, ester, and nanofluid generated from used cottonseed oil methyl ester, were aged (thermally aged). Breakdown strength, dielectric loss, dynamic viscosity, and density were among the tests carried out. These tests were run before and after the samples were aged, and the results were compared to the unaged sample. The optimal option was chosen based on the properties required for a mineral oil substitute.
Application of Nanoparticle/Nanofluids
Because of their excellent insulating properties, Nanofluids are used as coolants in high-power equipment like transformers. The high degree of heat generation in modern electronic devices presents issues for thermal management; as a result, nanofluids reduce system heat loss. The efficiency, weight, and complexity of thermal management systems can all be reduced by using nanofluids, which have the potential to significantly increase cooling rates for automobile and heavy-duty engines. Additionally, nanofluids reduce the amount of wear and friction on machine parts, saving more than 6 % of fuel. Many businesses employ nanofluids as a cooling agent since it reduces energy loss and emissions. Using nanofluids in heat exchangers can lower the volumetric and mass flow rates, reducing the amount of pumping power required to heat buildings, particularly in locations with the lowest temperatures. High-power-density light water reactors with pressurized water reactors (PWRs), emergency core cooling systems (ECCSs) of both PWRs and boiling water reactors, and coolants for in-vessel retention of the molten core after severe accidents all utilize nanofluids. Nanofluids improve the dependability and energy efficiency of designed systems, which lessens the wear and tear on automotive moving parts. Because of the positive characteristics of brake oils, nanofluids are used as vehicle brake fluids in hydraulic braking systems [24,25]. To lessen friction and wear between contacting surfacesmetal oxide, silicon, and carbon-based NPs have been added to lubricants [26]. When NPs are present, friction and wear are reduced due to the spherical NPs rolling or the nonspherical NPs sliding between tribopair surfaces [26]. When 0:5% ZnO or ZrO2 was added to the lubricant, for instance, the friction and wear of PAO-6 polyalphaolefin lubricant were reduced by 20 and 50 %, respectively [27]. In the production of integrated circuits (IC), abrasive NPs have been employed for silicon wafer chemical and mechanical polishing (CMP) [28]. To make nanocomposites with better mechanical properties, ceramic or carbon-based NPs have been introduced to polymer or metal matrices [28]. Light-emitting diodes, field-effect transistors, and organic photovoltaics have used semiconducting polymer NPs [29].
Materials and Method
Materials
In Funtua, Katsina state, Nigeria, 10 L of previously used crude cottonseed oil (UCSO) samples were purchased from a nearby restaurant. Inorganic scum was thereafter removed using filtration. The FFA contents of the UCSO samples were ascertained by utilizing a color indication titration method and adhering to ASTM D974 criteria. The oil does not require pretreatment because its FFA value is less than 2.5 %. In addition, the UCSO acid value was found to be 2.52 % and 1.26 %, respectively. There is a 3 % FFA limit for transesterification because, over this amount, the reaction undergoes hydrolysis and yields soap and water, which reduces the ester yield [30]. The natural ester used in this study has undergone rigorous purification, bleaching, and deodorization procedures. Sky Spring Nanomaterials, Inc. in the United States provided the factory-functionalized SiO2 nanoparticles employed in this study. Other materials include Methanol, Citric Acid, Sodium Hydroxide, Isopropyl Alcohol, Silica Hydrogel, Fuller Earth (Tonsil Supreme), and Isopropyl Alcohol, as well as Whatman No.5 and No.1, which were purchased from British Drug House Ltd (BDL) and Sigma-Aldrich both, were of analytical grade (Table 2).
Table 1. Previous studies on dielectrics ageing and applications

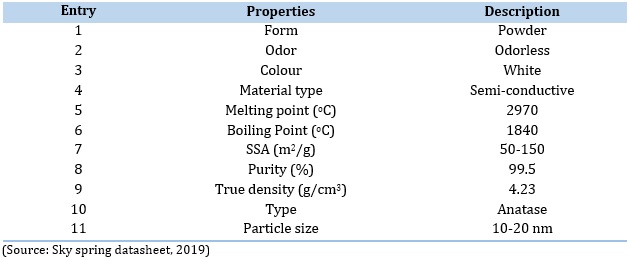
Table 2. SiO2 nanoparticle physical and chemical characteristics as found in the sky spring datasheet
Experimental Set‑ups
Thermal Ageing
In the current investigation, accelerated oil ageing was used to model the impact of heat degradation on the oil insulation of a transformer over a short time. This was done in laboratory circumstances. The pressboard was bought from Andrew Yule Pvt. Limited. Pressboard samples with dimensions of 4 cm × 4 cm × 2 mm were heated at 120 °C for 4 hours to remove any potential moisture. They were immersed in a mixture of mineral oil, ester, and nanofluid for 6 hours at 70 °C, respectively, to ensure adequate impregnation. A temperature-controlled reaction vessel containing 1 liter of mineral oil, UCSO-based natural ester, ester-impregnated pressboard, and copper was kept in a nitrogen atmosphere by continuously purging the vessel with nitrogen at a controlled pressure. The weight ratio of ester/pressboard/copper was maintained as 10:1:1. The upper limit for thermal ageing investigations is limited to 180 °C by IEEE guide C57.147 for acceptance and maintenance of natural ester fluids to prevent fluid scorching that would cause the watt density to increase and does not exceed 2 W cm−2 (12 W in−2) [32]. To reduce the impact of moisture, samples were removed from the thermal ageing process in the current analysis after 720 h at 120 °C and preserved in a desiccator. The analyses were done on Mineral oil, Ester, and CNF5, respectively.
Method
Purification
Purification was done in three stages on the UCSO: For acid degumming, citric acid was employed to dissolve the gum and phospholipids in the oil; 200 cm3 of warmed oil was combined thoroughly with citric acid using a magnetic stirrer for 20 min in a round bottom flask heated to 70 °C. The oil was then alkali neutralized using NaOH to lower the mixture's free fatty acids. For an additional 30 min, the NaOH solution was added, and the mixture was run. To remove the water content, the oil and mixture were oven-dried in a vacuum oven for 30 min. To bleach the oil and get rid of metals, colorful compounds, and oxidation byproducts, Tonsil Supreme (Fuller Earth) was utilized. This was achieved by swirling the liquid with a magnetic stirrer for 45 min after adding 5 g of fuller earth. Whatman Nos. 1 and 5 were then used to filter the mixture in the oven.
Transesterification
In a conical flask, 200 cm3 PCSO or Purified Cottonseed Oil was heated to 60 °C. The prepared oil was mixed with sodium methoxide and kept at a constant temperature for an hour (60 °C). The methoxide solution dissolved 2.7 g of anhydrous NaOH in methanol. After an hour, the solution was put into a separating funnel to properly separate the ester and glycerol. Afterward, warm water was used to wash the ester to remove any extra NaOH. To get rid of the methanol and water molecules from the sample, it was then dried in a vacuum oven at 80 °C.
Drug-Likeness, Pharmacokinetics, and Toxicity Assessment
Nanofluid is thought to be stable if the particle size is smaller than 100 nm [33]. There is still an agglomeration of nanoparticles at this particular size range because of an attraction force between the nanoparticles, especially when they are not functionalized. To get around this significant barrier, oleic acid is frequently utilized as a chemical to be adsorbed as a carboxylate on the surface of nanoparticles, enhancing the interfacial zone of the nanofluid as a solid-liquid suspension. The fluid interface zone produces intermolecular forces. According to the Van Der Waals theorem, intermolecular interactions prevent molecules from aggregating by keeping them separate. To produce the nanofluids, the functionalized nanoparticles (NP) were dispersed into fatty acid methyl ester at concentrations ranging from 0.1 wt. % to 0.8 wt. % with 0.1 wt. % increments. The sample descriptions are shown in Table 3. To compare pure virgin mineral oil to vegetable oil ester and nanofluid, pure virgin mineral oil is also purchased. A preliminary analysis and comparison of pure virgin transformer oil and vegetable oil with nanoparticle addition were conducted. These samples were tested before being aged. Breakdown strength, dielectric loss, dynamic viscosity, moisture content, and density tests are among the tests carried out. They were then aged thermally for 720 h within the ageing chamber (the temperature employed was 120 °C). The tests mentioned above were repeated to verify for changes in the qualities of the samples after ageing. A comparison of the results of the samples before and after ageing was conducted, and a better nano-material was suggested. After a complete analysis of the properties of the oil, the best possiblealternative to the mineral oil was chosen (Table 6).
Table 3. Samples Description

Results and Discussion
Fourier Transform Infrared Analysis
The presence of SiO2 nanoparticles is confirmed by the FTIR transmittance spectrum (400 to 4000 cm-1), as illustrated in Figure 1. Strong bands at 1073 cm-1 and 805.1 cm-1 were linked to Si-O-Si stretching vibration bonding that was both symmetric and asymmetric from the FTIR spectrum of the SiO2 nanoparticles [34]. The characteristic bands at 1085, 800, and 460 cm-1 are caused by the Si-O bonds stretching, bending, and moving out of the plane, respectively. The major Si-O vibrational band at 1073.5 cm-1, as well as its position and form, reveal the stoichiometric structure of SiO2 nanoparticles. The band between 461 cm-1 and 476 cm-1 is linked to a network of O-Si-O bending vibration modes, as was also observed by [35] in a similar study.
XRD Analysis
Strong peaks noticed at 70 ° and 90 ° in Figure 2 show that the sample contains more crystalline silica than average. The broad X-ray diffraction pattern in Figure 2 is typical of the nature of crystalline solids nanosilica [36,37]. From the Debye-Scherer in equation (1), the average crystallite size of the nanoparticle was estimated and found to be 11.2 nm; this was similar to what [38] obtained in similar research. The radiation of wavelength (λ=1.54059Å) at 40 kV and 30 mA. The data was taken for the 2θ range of 5 o to 160 o. The average crystallite size was calculated based on the X-ray wavelength, λ (1.54059 Å), and the peak breadth, β in radians through the Scherrer equation as shown in equation (1) [39,40]. Additionally, the nanoparticles' morphology and elemental compositions were determined using SEM with a ZEISS EVO LS10 operating at 20kV.
![]()
SEM – EDX Analysis
Shape and size variation, aggregation, homogeneous distribution, and tapering dispersion range were noted. High abundances of quasi-spherical forms were found in the SiO2 nanoparticles [41]. The elemental compositions of the nanoparticles were further studied using energy-dispersive X-ray spectroscopy (EDX) (JEOLJSM-600F), and the EDX spectra were recorded as shown in Figure 3 ; the two peaks of Si (89.73 %) and O (10.27 %) provides proof of the purity of the nanoparticles. Particle agglomeration was the cause of some observed irregularities in the SEM micrograph of the SiO2 nanoparticles.
Water Content
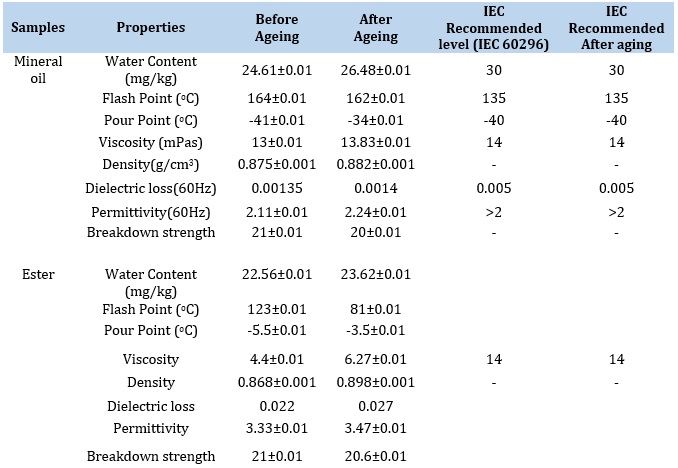
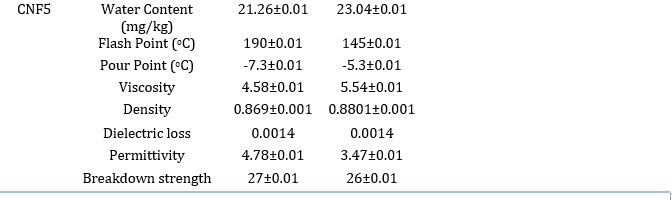
It is a method of determining how many water droplets are present in the samples. This property is essential since sample moisture concentration affects breakdown strength. As the water content of the sample increases, its dielectric strength decreases. The water content of samples typically rises as they get older and are used more frequently [42]. When moisture is present, the oil breakdown strength is decreased. The conductivity of water, which lowers the dielectric strength of the samples, is what causes leakage current flow. Table 6 demonstrates that the water content before ageing was lower than the recommended value. However, moisture is added to the samples as they age, impairing their ability to act as insulators. Table 6 also reveals that the water content did not rise above the recommended range even after the samples accelerated aging. CNF5 displayed the least amount of water content among all the samples both before and after ageing. More specifically, moisture infiltration in oil by ageing significantly accelerates the hydrolysis of hydrocarbon bonds. The ageing temperature significantly influences the equilibrium moisture distribution. The mineral oil aged at 120 °C has the maximum moisture content, as shown in Table 6. The polarization and depolarization processes are impacted by increased moisture content, which increases its permittivity and dielectric loss (Figs. 4.0-5.0). The dielectric breakdown strength of natural ester oils is reported to be strong for moisture contents up to 350 mg/kg. Ageing at 120 °C and above exhibits considerably greater moisture content in the current study, which can account for the decrease in Corona Inception Voltage values as reported by [42,43]. a similar study has noted that aged ester oil has increased acid content and relative moisture, which lowers the dielectric strength.
 Figure 1. Fourier Transform Infrared analysis of SiO2
Figure 1. Fourier Transform Infrared analysis of SiO2
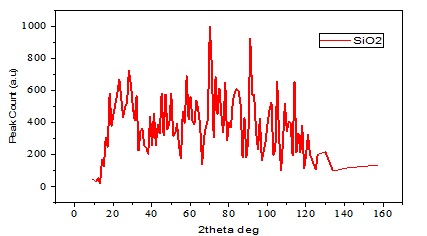
Figure 2. XRD Analysis of Nano SiO2
 Figure 3. Micrograph and EDX of inorganic SiO2 nanoparticle
Figure 3. Micrograph and EDX of inorganic SiO2 nanoparticle
Flash Point
The liquid flash point is the highest temperature at which a liquid sample ignites and emits a flash. If the flash continues for longer, it may harm some transformer components and cause the liquid to catch fire. So that the transformer can be thermally stable, a high flash point in the case of insulation liquid is necessary [44]. High flash point insulating oil is necessary for efficient insulation of power equipment, especially those used in residential areas, to minimize fire outbreaks. Utilizing oil with a high flash point is also necessary because a transformer failure could interrupt the power supply, leading to financial losses, exorbitant replacement prices, and labor-intensive repairs. The oil's flash point estimated following the American Society for Testing and Materials (ASTM) D93 standard [45-46]. Transesterification of the used cottonseed oil reduced the flash point of the esters from 242±0.01 °C to 123±0.01 °C. Flash points always decrease after transesterification [47]. However, mineral oil's flash point is still lower than 140 °C. Furthermore, the result is lower than the reported value [48]. They determined the flash point of natural palm ester to be 148 °C. It was observed that adding nanoparticles to the natural ester increased the flash point of the oil samples. More so, it was shown that the addition of SiO2 nanoparticles gradually elevated the flash point of the natural ester to 190±0.01 °C. The addition of 0.8 wt.% of SiO2 nanoparticles increased the flash point by 33.33 %.
Consequently, the presence of nanoparticles may have prevented the base oil triglycerides from dissociating, raising the flash point [49]. The flash points for natural esters and nanofluids fall within the guidelines for mineral oil-based insulating fluids. Nanofluid samples have a higher flash point, which is helpful since the thermal stability of fluid at high temperatures increases with increasing flashpoints. This confirms the capability of nanofluids as a superior substitute for mineral oil in liquid-filled power transformers for interior and nearby building installations. Moreover, the flash point of diesel fuel is a common indicator of fuel flammability or nonflammability behavior [50]. Gasoline can only ignite at this temperature. As per ASTM D93, the minimum temperature is 130 °C.
Nevertheless, the result is lower than the reported 164 °C [51]. This establishes the safety of handling, storing, and using biodiesel produced from used cottonseed oil in engines and transformers. It is not flammable, either. According to [52] non-hazardous substances have flash points of 93 °C or higher. The biodiesel that is produced is significantly safer than regular diesel. The high flash point found reveals that the biodiesel produced is virtually methanol-free, as even minute levels of methanol can dramatically lower the flash point and negatively affect fuel pumps, seals, and elastomers [53]. Similar results were also reported by [22].
Pour Point
The lowest temperature at which a liquid loses its characteristics and solidifies into a semi-solid state is known as the pour point. Mineral oil loses its ability to flow once the pour point is reached, which could result in failures. As a result, the mineral oil pour point should be as low as possible since the liquid becomes semi-solid at that temperature and transformer efficiency suffers [4]. The fact that accelerated ageing deteriorates the thermal properties of the oil can be attributed to the increase in pour point values for all oil samples. The natural ester pour point was -5.5±0.01 °C, while SiO2 nanofluids were found to have a 33 % higher pour point at 0.8 wt. % concentration, even though this property of the oils is not within the recommended level. The results of the pour point measurement are listed in Table 6 below. These results were lower than -4 °C pour point of natural ester synthesized from palm kernel oil [54].
Dynamic Viscosity
Table 4 displays the dynamic viscosity of the aged samples. The samples underwent accelerated ageing at 120 oC for 720 h. The dynamic viscosity of ester increased after ageing. However, the presence of nanoparticles in the base fluid stopped the dynamic viscosity from increasing relative to the base fluid. The rheology of the oil samples was studied to see how nanoparticles affected thermal ageing and how well the oil performed after thermal ageing. After the oil samples have been aged for 720 h at 120 oC within the ageing chamber, their dynamic viscosity increases. More so, the dynamic viscosity of methyl ester increases by 42.50 % after thermal ageing. However, it still has a lower dynamic viscosity than mineral oil that has not been aged. This suggests that aged methyl ester can still function admirably as a coolant. Triple unsaturated fatty acids cause low initial dynamic viscosity in the oil sampleith low oxidation stability [55]. Despite the claim that sludge does not form during thermal ageing in ester, oxidative breakage of unsaturated bonds causes a minor increase in dynamic viscosity as oil ages. This increases, especially at higher temperatures. A similar study [54] determined the viscosity of ester from palm kernel oil to be 4.12 mPas. It was observed that the viscosity of the oil increases at every loading of TiO2 nanoparticles (0.2 wt.% to 1 wt.%) at temperatures 30 °C, 40 °C, and 50 °C. This value is below 6.27 mPas obtained in this study.
Density
After ageing, the density of the used cottonseed methyl ester slightly increases. Ageing does not significantly affect the density of the nanofluid, though. While a modest increase in density was noted, it can be explained by high molecular weight ageing products. These products contain contaminants or other impurities. The equation (2) below has been used to determine a relationship between viscosity and average molecular weight for polymeric materials [56].
![]() Where; [μ] is the viscosity of the polymeric material, K and α are constants that depend on solvents and temperature. From this equation (2), the dynamic viscosity is proportional to the molecular weight of the oil. This suggests that the rise in the viscosity of the oil is caused by an increase in the molecular weight of the oil as it ages. According to the nanoparticle loading percentage, nanoparticles slow down the rate at which oil molecules degrade by absorbing the heat that causes this. This prevents the methyl ester from polymerizing and stops the density and viscosity from increasing. The produced nanofluid density and viscosity are barely affected by thermal ageing. The UCSO-based methyl ester and nanofluid viscosity were found to be lower after ageing compared to the required value as per the IEC standard (12 mPas) (Tables 5.0 & 6.0). Since low-viscosity oil is preferred for insulation, it suggests that nanofluid will continue to function well as a coolant in high-voltage insulation even after ageing. Similar observations were made by [54].
Where; [μ] is the viscosity of the polymeric material, K and α are constants that depend on solvents and temperature. From this equation (2), the dynamic viscosity is proportional to the molecular weight of the oil. This suggests that the rise in the viscosity of the oil is caused by an increase in the molecular weight of the oil as it ages. According to the nanoparticle loading percentage, nanoparticles slow down the rate at which oil molecules degrade by absorbing the heat that causes this. This prevents the methyl ester from polymerizing and stops the density and viscosity from increasing. The produced nanofluid density and viscosity are barely affected by thermal ageing. The UCSO-based methyl ester and nanofluid viscosity were found to be lower after ageing compared to the required value as per the IEC standard (12 mPas) (Tables 5.0 & 6.0). Since low-viscosity oil is preferred for insulation, it suggests that nanofluid will continue to function well as a coolant in high-voltage insulation even after ageing. Similar observations were made by [54].
Dielectric Response of Aged Oil Samples
As shown in Figure 4, the relative permittivity associated with the energy held in the aged samples was determined at a power frequency of 60 Hz. The relative permittivity of the oil samples increased as the ageing period increased. The production of high molecular mass polymeric components may be the cause of this evolution. When exposed to an electric field, these impurities in the oil become polarized, increasing the net polarization in the oil. The rate of increase in the dielectric constant is well pronounced in the base oil due to the absence of nanoparticles. These particles could have prevented the thermal degradation of the ester. The graph in Figure 4 shows that the dielectric constant of the nanofluid increases. Nevertheless, the increase should be viewed as inconsequential since nanoparticles are present and inhibit the formation of polymeric materials [19]. A similar observation was recorded by [22] and [54] in a similar study.
Table 4. Dynamic Viscosity of the Oil Sample Subjected to Accelerated Ageing at 120 oC for 720 hours

Table 5. The Density of Oil Samples Subjected to Accelerated Ageing at 120 oC for 720 hours

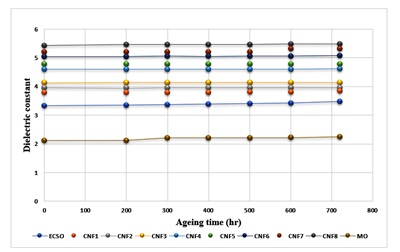 Figure 4. Effect of ageing time on the Relative Permittivity of MO, ECSO, and Nanofluids
Figure 4. Effect of ageing time on the Relative Permittivity of MO, ECSO, and Nanofluids
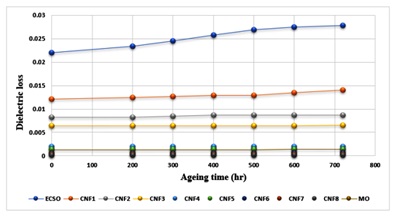 Figure 5. Effect of Ageing Time on the Dielectric Loss of MO, ECSO, and Nanofluids
Figure 5. Effect of Ageing Time on the Dielectric Loss of MO, ECSO, and Nanofluids
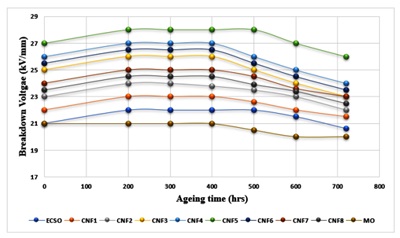 Figure 6. Effect of Ageing Signature on the Breakdown Strength of MO, ECSO, and Nanofluids
Figure 6. Effect of Ageing Signature on the Breakdown Strength of MO, ECSO, and Nanofluids
Table 6. Compares Some Basic Properties of Unaged and Aged Mineral Oil, Ester, and Nanofluid with the IEC Standard
Dielectric Loss
Figure 5 depicts how ageing has affected the dielectric loss of the oil samples. As the samples aged, the dielectric loss of the oil sample increased a little compared to that of the base oil, which increased more noticeably. The dielectric loss of the samples was measured at the frequency of 60 Hz, which is the operating frequency of an in-service transformer. The evolution of ageing was visible in the base oil. As aging time increases through constant temperature, so does the dielectric loss of the base oil. The ageing process causes some contaminants incorporated during the polymerization process to produce more ionic charge carriers, leading to increased ionic conduction losses [57-60]. As was previously said, the nanoparticle content of the oil prevented the production of polymeric materials over time. This prevented an increase in dielectric loss of the oil over time. Also, the aged oil contains nanoparticles that can trap ionic charge carriers, preventing ionic conduction. Hence, the AC conductivity of the base oil is related to the dielectric loss, and as methyl ester ages, its conductivity increases. The increase in conductivity, however, was prevented by the ongoing nanoparticle loading of the base oil. From Table 6, when the loading of the nanoparticle increases, the influence of thermal deterioration also increases conductivity, as observed in a similar study [22], [54], [57], [61-63].
Breakdown Voltage of the Aged Oil Samples
Mineral oil, methyl ester, and nanofluids exhibit a similar ageing tendency. The dielectric breakdown strength of the oils increased as a result of the thermal ageing of the oils between 0 and 400 hours. This can be due to the moisture content of the oil getting lowered. The water content in the oil can be ionized and causes conduction in the oil, which may lead to a breakdown in an electric field. Also, the breakdown strength increase might result from a chemical change in the liquid. This chemical change may have made the liquid between the electrodes more resistant to breakdown. It was observed that further ageing of the oil led to a decrease in the breakdown strength of the oil [62,64]. When the heat loss could not balance the heat gained by the liquid, an exponential increase in conductivity occurred, leading to an accelerated breakdown mechanism. By implication, it indicates that the ageing products, which have increased with ageing, have reduced reakdown voltage due to conduction in the system [11]. The breakdown strength of the oil after ageing is unaffected by the nanoparticles' loading. According to Tables 6 and Figure 5, a 0.5 wt. % loading of nanoparticles in oil increases reliability. This oil will work well as insulating oil in voltage equipment because its breakdown strength is much higher than mineral oil after accelerated ageing [65]. This was similar to the results obtained for wet coconut oil and wet and dry transformer oil aged in sealed and unsealed conditions at 1200C [21,22] using CCTO(CaCu3Ti4O12) nanopowder and also the results obtained by [54].
Conclusions
To improve the thermal and dielectric properties of the ester, nanoparticles were added, and the SiO2 nanoparticle that was chosen had already been functionalized from the factory. Water content, flash point, pour point, viscosity, density, dielectric loss, permittivity, and breakdown strength tests were conducted on the samples before and after ageing. Accelerated multi-aging was performed on oil samples for 720 hours. Results revealed that adding 0.8 wt. % of SiO2 nanoparticles to CNF5 improved performance even after samples were aged. CNF5 outperformed the other samples in terms of both thermal and dielectric property improvement. In the case of CNF5, breakdown voltage, flash point, viscosity, tan delta, pour point, and water content was lowered. Employing FTIR, XRD, and SEM, the functional group, crystallite size, and surface shape of the nanoparticles were all identified. It was evident that the dispersion of SiO2 nanoparticles in the methyl ester enhanced the physicochemical characteristics of the natural ester. To do this, nanofluid samples, both aged and unaged, exhibit improved values for electrical attributes such as breakdown voltage and loss tangent compared to the base oil samples. Physical characteristics like viscosity and pour point exhibit good results, that is, the decrease in the characteristics due to greater nanoparticle concentration. Both aged and unaged samples exhibit increased thermal stability, as evidenced by their flash points. Increased particle volume fraction exhibits improved flash point values as a result.
Acknowledgment
The authors gratefully acknowledge the management of the Multi-User Science Research Laboratory at Ahmadu Bello University in Zaria for providing the laboratory space necessary for this investigation.
Disclosure statement
The authors reported no potential conflict of interest.
Orcid
Abdullateef Jimoh : 0000-0002-3240-9682


