Document Type : Original Research Article
Authors
Department of Chemistry, Payame Noor University (PNU), P.O.Box 19395-4697, Tehran, Iran
Abstract
In the present study, a simple and rapid method using an effective heterogeneous nanomagnetic catalyst, Fe3O4@SiO2-SO3H, was used to prepare the 2-arylbenzoxazoles derivatives by a condensation reaction of aromatic aldehydes with 2-aminophenol without using solvent and at a temperature of 50 0C. This catalyst is magnetically recycled and reused several times without reducing its catalytic activity. It was demonstrated that Fe3O4@SiO2-SO3H is an economic catalyst that can be used several times without reducing the catalytic properties. Purifying of the final products was simple and easy, and the percentage of catalyst performance was satisfactory.
Graphical Abstract
Keywords
Main Subjects
Introduction
The number of bacterial infectious diseases resistant to several drugs is increasing. Also, doctors are trying to find reliable antibiotics for severe infections. Therefore, there is still a need for new classes of drugs with antimicrobial properties, including benzoxazole derivatives.
Compounds of heterocyclic benzoxazole and its derivatives are particularly interesting to chemists because they exist in natural and biologically active compounds. Benzoxazole derivatives show many biological and medicinal activities such as anti-cancer, anti-HIV, anti-fungal, anti-microbial, anti-bacterial, and anti-viral activities [1]. Many methods have been proposed to prepare benzoxazole derivatives; the two standard methods are the reaction of 2-aminophenol with carboxylic acid derivatives [2, 3] and the compression reaction of 2-aminophenol with aldehydes in the presence of various catalysts [4-14]. Usually, these methods have many disadvantages, including using organic solvents harmful to the environment, many catalysts, harsh reaction conditions, and long reaction times. As a result, there is still a need to develop green, useful, easy, and mild methods for preparing benzoxazole derivatives.
Compared to homogeneous catalysts, heterogeneous catalysts have less selectivity and activity due to their insolubility in the reaction medium. Since these catalysts' chemical and thermal stability is low, it has limited their performance, and these catalysts can hardly be separated from the reaction mixture [15-17].
One of the most common methods, in this case, is stabilizing insoluble materials with homogeneous catalysts, which has many benefits, such as easy catalyst recycling, excellent resistance, and stability against degradation [18, 19]. These nanoparticles (MNPs) can be useful solid materials for supporting homogeneous catalysts due to their easy processing, variety, and high surface area. Considering the many applications of MNPs and their stabilization on homogeneous organic catalysts, and their heterogenization in chemistry, medicine, materials science, and catalysis, it has been widely studied in academic centers and industries [20]. These compounds are used for the heterogenization of catalysts due to having large surface area, variety, easy processing, and availability. This type of environmentally friendly, stable, active, and reusable nanocatalyst is significant in green chemistry [21-23].
Following the research for the preparation of heterocyclic compounds [24-28], we now report the preparation of 2-arylbenzoxazole by the reaction of aryl aldehydes and 2-aminophenol using the Fe3O4@SiO2-SO3H catalyst as an effective solid acid magnetic nanocatalysts reusable in solvent-free conditions.
Experimental
The materials used in this research were procured from reputable Merck and Aldrich companies. Thin layer chromatography (TLC) was used to investigate the progress of the reaction. All the prepared products were determined by comparing their physical properties with authentic samples. Using electrothermal device 9200, the melting point of the products was measured in open capillary tubes. Using a Shimadzu IR Prestige-21 spectrophotometer, the spectrum of the synthesized compounds was taken, and the synthesized compounds were identified by analyzing the spectrum of the functional groups. XRD spectrum was taken using PANalytical X'Pert Pro x-ray diffractometer, and the crystal structure of nanoparticles was determined by spectrum analysis. Nanoparticle VSM was determined at room temperature at Magnet Sahara Company, Kashan, Iran.
Preparation of the catalyst
To prepare Fe3O4 superparamagnetic nanoparticles, FeCl2•4H2O (6.37 g, 32 mmol) and FeCl3•6H2O (15.15 g, 56 mmol) were dissolved in 640 ml of deionized water and kept under nitrogen at 80 °C for 11 hours. It was stirred, and adding a 25% ammonium hydroxide solution (80 mlmL) was vigorously stirred for 1 hour. The sediment particles formed were separated by magnetic discharge, washed several times with hot water, and vacuum dried at 70 °C.
Fe3O4 MNPs (2 g) were dispersed in a solution of ethanol (450 mL) and water (120 mL) and ultrasonicated for 25 min. Then 10 mL of 25% ammonium hydroxide and 2 ml of tetraethyl orthosilicate (TEOS) were slowly added, and the reaction mixture was stirred for 12 hours. A magnetic discharge separated the silica-coated magnetic nanoparticles, washed several times with deionized water, and dried under vacuum at 60 °C overnight.
Fe3O4@SiO2 (2 g) was added to CH2Cl2 (75 mL) and ultrasonicated for ten minutes. Then, dropwise was added to the reaction mixture by chlorosulfonic acid (1.75 g, 15 mmol) diluted in 20 mL of CH2Cl2. The reaction mixture was stirred at room temperature, and the remaining HCl was removed. Finally, sulfuric acid Fe3O4@SiO2 was separated from the reaction mixture by an external magnet, washed several times with dry CH2Cl2, and dried at 60 °C under vacuum [29].
The usual method for the synthesis of 2-arylbenzoxazoles using Fe3O4@SiO2-SO3H catalyst
0.03 g of catalyst was added to the mixture of 2-aminophenol (1 mmol) and aromatic aldehydes (1 mmol), and the reaction mixture was stirred at 50 °C without solvent for the necessary time (Table 2). After the reaction, as indicated by TLC (1:3 n-hexane ethanol), hot ethanol (3 mL) was added to the mixture and stirred for 5 min. Using an external magnet, the magnetic nanocatalysts were removed. It was recrystallized with hot ethanol, and then the pure product was isolated. Its purity was confirmed by determining and comparing the physical data with valid examples.
Magnetic nanocatalysts (Fe3O4@SiO2-SO3H) were prepared by a brief method shown in Scheme 1. By co-precipitation of FeCl2.4H2O and FeCl3.6H2O, magnetite nanoparticles were synthesized in a basic solution by hydrolysis catalyzed by tetraethylorthosilicate ammonia. Proper silica deposition on the surface of nanoparticles and, finally, SiO2 spheres were functionalized by SO3H groups through a mixture of core-shell magnetic nanocomposite and chlorosulfonic acid in dry CH2Cl2.
 Scheme 1. Preparation method of Fe3O4@SiO2-SO3H magnetic nanoparticles
Scheme 1. Preparation method of Fe3O4@SiO2-SO3H magnetic nanoparticles
Results and discussion
FT-IR spectra of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2-SO3H are demonstrated in Figure 1. In the FT-IR spectrum of the catalyst, the stretching vibration of Fe-O shows it as a strong band at 602 cm-1. Asymmetric stretching, symmetric stretching, and Si-O-Si bending vibrations are observed at 1069, 999, and 471 cm-1, respectively. The symmetric and asymmetric stretching absorption bands of sulfonic acid functional group O=S=O are confirmed at 1142-1219 cm-1. The broad peak at 3410 cm-1 related to O-H stretching absorption and O-H deformation vibration near 1636 cm-1 confirms the presence of SO3H fragments in the catalyst structure.
The SEM images of Fe3O4@SiO2-SO3H shown in Figure 2 confirm that the length of the synthesized nanocatalysts is less than 50 nm, and the prepared nanocatalysts have an almost spherical morphology.
The EDS result obtained from the SEM interpretation of the catalyst is shown in Figure 3. According to Figure 3, there are elements of oxygen, iron, sulfur, and silicon in the structure of the catalyst.
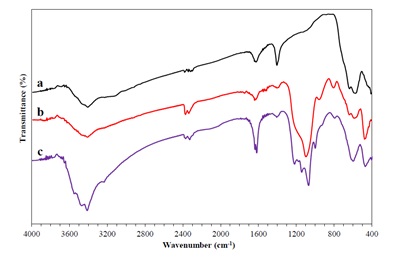
Figure 1. Infrared spectra of (a) Fe3O4, (b) Fe3O4@SiO2, and (c) Fe3O4@SiO2-SO3H

Figure 2. SEM images of Fe3O4@SiO2-SO3H
 Figure 3. EDS spectrum of Fe3O4@SiO2-SO3H
Figure 3. EDS spectrum of Fe3O4@SiO2-SO3H
Room temperature magnetic properties of the bare Fe3O4, Fe3O4@SiO2 core-shell nanostructure, and Fe3O4@SiO2-SO3H nanocatalyst were investigated by a vibrating sample magnetometer (Figure 4). The magnetic scheme shows that the saturation magnetization of magnetic iron oxide is (66 emu/g) and Fe3O4@SiO2 (42 emu/g), while the saturation magnetization of the new catalyst is 19 emu/g. The presence of non-magnetic materials on the surface of nanoparticles reduces the magnetic saturation of the catalyst.
XRD analysis shows the crystallinity angle of magnetite nanoparticles in the prepared catalyst to be 10-80° in the 2θ region (Figure 5). According to the XRD spectrum, the intensity and position of the diffraction peaks at 2θ = 30.24°, 35.62°, 43.26°, 53.56°, 57.26°, and 62.90° are completely consistent with the Fe3O4 spectrum (2θ = 30.24). 35.69°, 43.32°, 53.81°, 57.26° and 62.92°) [30].
Comparing the magnetic curve of catalyst Fe3O4 (42 emu/g) and catalyst Fe3O4@SiO2-SO3H (42 emu/g), we conclude that the decrease in the magnetic saturation of the catalyst is due to the presence of the non-magnetic material of the silica shell on the surface of the nanoparticles.
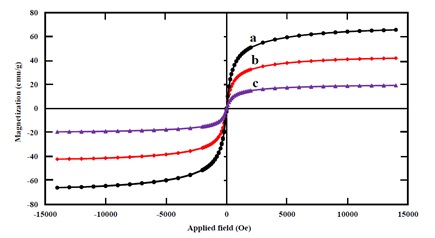 Figure 4. The magnetization curves of (a) Fe3O4, (b) Fe3O4@SiO2, and (c) Fe3O4@SiO2-SO3H
Figure 4. The magnetization curves of (a) Fe3O4, (b) Fe3O4@SiO2, and (c) Fe3O4@SiO2-SO3H
 Figure 5. XRD spectrum of Fe3O4@SiO2-SO3H
Figure 5. XRD spectrum of Fe3O4@SiO2-SO3H
The catalytic activity of Fe3O4@SiO2-SO3H nanocatalysts was investigated to prepare benzoxazole derivatives. The reaction of 2-aminophenol with benzaldehyde was studied as a model reaction to optimize the reaction conditions. After the initial tests, the best result was related to the reaction without solvent and the amount of .03 g of catalyst. (Table 1, entry 10). Table 1 shows that the reaction does not take place without the presence of a catalyst. The reaction speed increases by increasing the amount of catalyst, and the complete product is obtained by using 0.03 g of catalyst in the reaction. More catalyst has no significant effect on reaction time or product efficiency. Meanwhile, by increasing the reaction temperature, it is determined that the reaction speed is proportional to the increase in temperature, and the best temperature is 50 °C.
Table 1. Optimization study of the reaction conditions in the condensation of benzaldehyde with 2-aminophenol

These conditions were investigated for converting aromatic aldehydes with electron-donating and withdrawing groups to their respective benzoxazole (Scheme 2, Table 2).
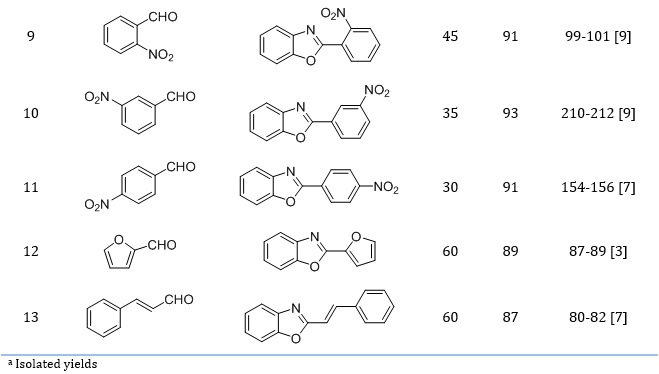
Table 2 shows that all the studied aryl aldehydes were quickly converted into 2-arylbenzoxazoles with high efficiency.
 Scheme 2. Synthesis of benzoxazole using O-aminophenol and different aldehydes in the presence of Fe3O4@SiO2-SO3H
Scheme 2. Synthesis of benzoxazole using O-aminophenol and different aldehydes in the presence of Fe3O4@SiO2-SO3H
Table 2. Synthesis of 2-arylbenzoxazoles catalyzed by Fe3O4@SiO2-SO3H


The condensation reaction of benzaldehyde and 2-aminophenol at a temperature of 50°C was investigated for the possibility of recycling the catalyst in solvent-free conditions. After each reaction, the catalyst was separated from the reaction mixture, washed with ethanol and acetone, dried in an oven at 60°C for one hour, and reused in subsequent reactions. The small decrease in product yield (92%, 90%, 87%, 86%, and 84% in cycles 1-5, respectively) indicates that the catalyst can be recycled and reused at least five times without an apparent decrease in its performance (Figure 6).
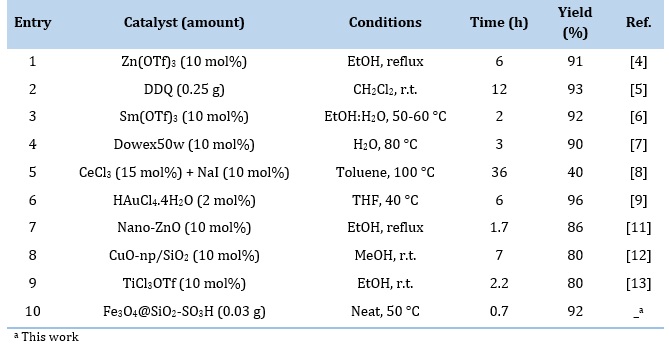
The catalytic efficiency of Fe3O4@SiO2-SO3H in condensation of 2-aminophenol with benzaldehyde was compared with several other catalysts. As shown in Table 3, the present method overcomes the previously reported methods are superior due to shorter reaction time, higher yield percentage, catalyst loading rate, and compatibility with the environment.
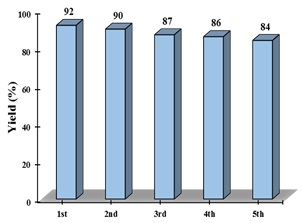 Figure 6. Reusability of the catalyst
Figure 6. Reusability of the catalyst
Table 3. A comparative analysis of the effectiveness of different catalysts for preparing 2-pheylbenzoxazole
Conclusion
As a result, Fe3O4@SiO2-SO3H nanoparticles were successfully synthesized and identified by different methods. Then the prepared nanocatalysts were used to synthesize 2-aryl benzoxazole through the condensation reaction of different aromatic aldehydes with 2-aminophenol. Compared to the previously reported methods, this method has many advantages, such as reducing reaction times, higher efficiency, simplicity of the reaction, avoiding dangerous acids or bases, environmentally friendly, simple separation of the nanomagnetic catalyst, and reusing it without reducing its activity.
Acknowledgment
In this research, we benefited from the financial support of Payame Noor University.
Disclosure statement
The authors declare that they have no conflict of interest.