Document Type : Review Article
Authors
- Emmanuel Ibukun Aduloju 1, 2
- Noorfatimah Yahaya 2
- Nadhirah Mohammad Zain 2
- Mohammad Anuar Kamaruddin 2
- Muhammed Ariffuddin Abd Hamid 2
1 Department of Science Technology, School of Applied Science and Technology, The Federal Polytechnic Offa, P.M.B. 420, Kwara State, Nigeria
2 Department of Toxicology, Advanced Medical and Dental Institute (AMDI), Universiti Sains Malaysia, 13200 Bertam Kepala Batas, Penang, Malaysia
Abstract
Preparing the sample is a vital procedure involving liquid and solid handling approaches toward extracting, enriching, and identifying target analytes from complex environmental matrices. Thus, there is a need for more effective quantitative investigation and green evaluation procedures better than the conventional techniques for the accurate assessment, optimum selection, and testing of analytical techniques. To achieve greenness in analytical methodology, performance criteria such as design procedures, quantities of safe solvent (ionic liquids (IL), deep eutectic solvents (DES)) /toxic reagents consumed, utilization of renewable, reusable and recyclable materials/ biomolecules were evaluated. In this review, economical and eco-friendly approaches are proposed towards an agreement with the novel principles of Green Analytical Chemistry (GAC) using the combination of figures, colorations, unified 0–1 scale, and graphical illustrations: these include National Environmental Methods Index (NEMI), Analytical Greenness Calculator (AGREE), Analytical Eco-Scale (AES), Green Analytical Procedure Index (GAPI) and Pictograms like Hexagon (H) amongst others. These metric tools were proposed and generally adopted to directly assess a systematic and comprehensive improvement of green sample pretreatment, preparation for different extraction, and recovery methods from various modes. These tools are also aimed at evaluating the sustainability of the methods through figures of merits, carbon footprint, and other GAC tools by assigning penalty points (0 to 4) for each block and hexagonal pictograms that provide an easy comparison between analytical procedures
Graphical Abstract
Keywords
- Sample preparation
- Analytical GREEness Calculator (AGREE)
- Green Analytical Chemistry (GAC)
- Analytical Eco-Scale (AES)
- Deep Eutectic Solvents (DES
- Ionic liquids (IL)
- Green Analytical Procedure Index (GAPI)
- Complex environmental matrices
Main Subjects
Introduction
Green chemistry involves using eco-friendly materials and nanocomposites and consciously managing the waste generated towards extracting and recovering targeted analytes from complex environmental matrices. This is the most significant difficulty researchers face in analytical chemistry. Hence diverse concepts characterized by the green chemistry approach have been created to make a more efficient and environmentally benign sample pretreatment and preparation approach. This approach, called GAC, involves sample collection, preparation, solvent/reagents (safe and toxic), analytical measurement, instrumentation, data evaluation, and general extraction and recovery method type. The GAC is vital to sample preparation owing to the cleanup, removal of interfering impurities towards the preconcentration, enrichment, and degradation of trace and ultra-trace target analytes from complex environmental matrices, thus improving the sensitivity, selectivity, and accuracy of electromigration, chromatographic and spectroscopic techniques. In addition, objective analytical performance parameters are investigated and evaluated as penalty points such as toxicity of solvent and reagents, carbon footprints, residue, the overall cost of the experiment, and figures of merit which are invariably scaled from 0 to 4.
Similarly, the overall results are depicted and converted to a regular hexagonal pictogram, providing a more user-friendly contrast between the analytical procedures. These evaluation tools are geared towards principles of green chemistry and revolve around the completeness of figures of merits obtained from optimization of problem-solving procedure, eco-friendliness, waste generated, time, solvents, apparatus used, and damages caused. These simple, fast, and precise tools include, National Environmental Methods Index (NEMI)(‘National Environmental Methods Index’, 2006), Green Certificate (GC), White Analytical Chemistry (WAC) [2], Green Analytical Procedure Index (GAPI)[3], Analytical GREEnness Calculator AGREE[4], Pictograms like Hexagon (H) [5], HPLC-EAT (Environmental Assessment Tool)[6], Analytical Method Volume Intensity (AMVI)[7], Analytical Method Greenness Score (AMGS) Calculator [8,9] and Analytical Eco-Scale[10], have been employed to investigate and evaluate the GREEnness of any approach used. The analytical greenness metric system for the preparation of samples (AGREEprep) [11, 12] could also be utilized as a sample pretreatment procedure by investigating the 10 criteria of Green Sample Preparation (GSP)[13]. The concept of greening chemical philosophy was first proposed, developed, and implemented by a renowned researcher Paul Anastas in 1998 as principles, guidelines, and recommendations for the scientific community [14-16].
This became a misconception considering that all the available extraction and recovery techniques are not without sample preparation (without any reagent or solvent) and are characterized by novel analytical performances, which are substantially better when compared with the actions of direct analytical techniques on complex environmental matrices. The recovery and extraction methods are divided into liquid-liquid and solid-phase extraction, which requires a small volume of solvents (miniaturization) via microextraction towards the separation and concentration of target analytes via mass transfer and partitioning before quantitative determination. Examples of these methodologies include HFME, SFO, DLLME, and SDME [17-20].
The importance of these metrics is supported by many citations in the Scopus databases (www.scopus.com). Until February 7, 2023, 144 and 86 citations for GAPI and AGREE from 2001 to 2023 and 2019 to 2023, respectively, were reported.

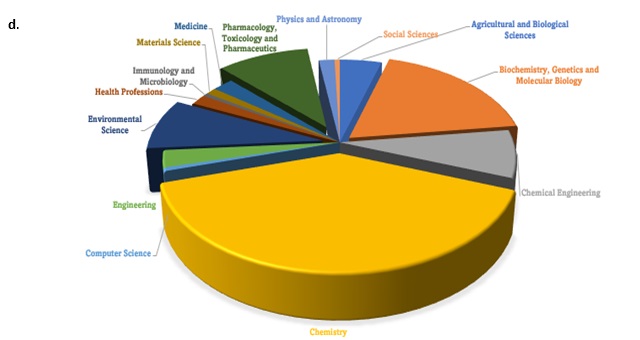
Figure 1. (a) Growing Numbers of Journal publications. (b) Journals Sources with GASC principles of extraction (c) subject areas of researchers whose journals reviewed (d) Countries where publications concerning GASC applications using the keywords “principles of green analytical sample preparation” (source www.scopus.com)
This can be inferred from the increasing number of research publications between 2019 to 2023, enabling, contributing, and empowering the impact of the greenness of GSP and GAC principles that encourages the adoption of eco-benign sample preparation actions concerning a sustainable future, as shown in Figure 1a-b.
Although, the impression created by the first principles of the GAC was that elimination of the sample preparation approach in any extraction and recovery method makes the process “green”, thus omitting and disregarding the greening advancement in technology of the method employed. The sample preparation approach is essential for the following: preconcentration of target analytes, conversion/transformation of complex solid sample into suitable and compatible for analytical instrumentation, selective extraction and recovery of target analytes from impurities, and cleanup of the sample. Also, the implausible assumptions did not consider the conversion of solid complex environmental matrices (plants, biomolecules, and food samples) into the suitable and compatible form necessary for electromigration, chromatographic or spectrometric analysis, that is where direction analysis of the sample is not probable. In addition, in the “dilute-and-shoot” methods, where the sample matrices (human fluids, tissues) are diluted serially from 1:1 to 1:100,50 (V/V) before direct analysis, it negatively impacts the sensitivity of the method [21,22]. However, there are conventional technological approaches in analytical instrumentation (electrospray ionization mass spectrometry) capable of partly defeating the matrix-related challenges and sensitivity issues affiliated with direct analysis, although the approach can be expensive [23]. Thus, instead of creating a gap with the omission of the sample preparation step, efforts should be geared towards ensuring that the self-same vital step is comprehensively defined within the Green analytical sample preparation concept (GASPC), a combination of GAC and GSP. Remarkably, the bottleneck created by sample treatment steps can be made suitable for greenness, thus improving the GAC and providing suitable, sustainable, and green alternatives characterized by strategies like miniaturization, online waste treatment, energy saving, and reduced consumables. Also, innovation and redefining the sample preparation step in line with GASPC will impact transitioning green effect to complex, global, and interrelated environmental challenges faced by analytical researchers and humanity, thus addressing sustainability and promoting GAC and, ultimately GSP.
The 12 Greenness principles of GAC
Summarily, the 12 principles gravitate towards utilizing catalytic methodologies, renewable feedstocks, the economy of chemical elements, and mitigation of the deleterious environmental impact of all chemical processes involved. This approach was based on the greenness of both analytical and synthetic chemistry techniques, which include reduction in Generated waste (PR 1), eco-friendly solvents, reagents, and auxiliaries (PR5), efficient energy consumption (PR6) and reduction in derivatization (PR 8), where PR is principle’s reference number.
As proposed by [24], the 12 principles of GAC are reinvented as follows:
- Direct analytical techniques should replace Sample treatment.
- The reduced particle size of the sample and micro amount in weight and volume
- Simultaneous analytical measurements should be incorporated.
- Integration of analytical approaches that reduce the consumption of solvents, reagents, and energy.
- Miniaturized and automated approaches should be inculcated.
- No Derivatization
- Reduction of analytical waste involving proper waste management procedures like reuse, recycling, and regeneration should be adopted.
- Suitable multi-residue determinations should be adopted per time against uni-analyte determination.
- The utilization of energy should be reduced.
- Preference for suitable reagents and solvents from a renewable source.
- Toxic reagents should be replaced /eliminated.
- Reduction of occupational hazard.
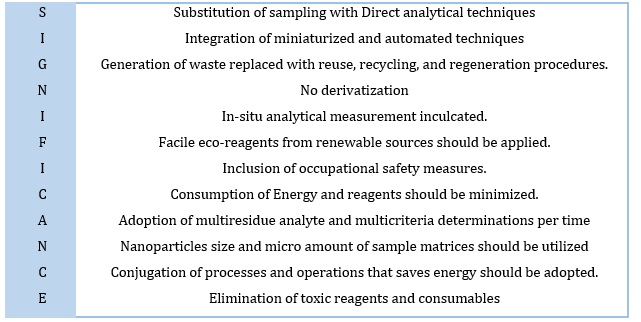
Subsequently, Galuszka et al., in 2013, formulated and summarized the 12 greening chemical philosophies into acronyms “SIGNIFICANCE” terms as shown in Table 1, which encourage the simultaneous application of analytical methods to avoid sample preparation. Also, any “green” process employed during the sample preparation step (miniaturized process, automation, reduction in a non-toxic solvent, reagent, energy consumption, multianalyte determinations, and occupational safety from hazards) was altering selectivity, sensitivity, accuracy, detectability, integrity, and precision of the process [24,25].
Table 1. Summarized principles of green analytical chemistry in Acronyms “SIGNIFICANCE” [24]
Six building blocks of Greenness principles of GAC
Research has shown that chemical analysis comprises complex processes involving numerous steps that can be replaced with suitable green alternatives. It is vital to investigate each approach and methodology in line with GAC's greenness principles, from sampling to characterization. These vital greenness principles are summarized in this review as follows: sample, reagents, analytical instrumentations, reduction in energy consumption, complete elimination of waste from consumables (solvents, reagents, additives, and preservatives), particle size reduction of sample matrices, proper management of analytical waste and enhancement of occupational safety as illustrated in Figure 2.
 Figure 2. Building Blocks of Greenness of GAC principles
Figure 2. Building Blocks of Greenness of GAC principles
Sample and sampling
The greenness of any analytical approach should be reflected in the use of a minimal number of samples and the reduced particle size. However, the application of direct analytical techniques to samples should be encouraged. To reduce the number of samples and sample size, statistical data and a noninvasive approach should be employed. Examples include remote sensing, magnetic surveys, seismic techniques, and portable or hand-held instruments like XRF[26–28]. While the former involves the use of reliable statistical data (e.g., barbell cluster ANOVA design) obtained from sampling sites [29]. It is also essential to employ miniaturized analytical operations [30] or green sample induction systems (LA-ICP-MS), characterized by the inherent potential of absolute sample representation even when dealing with heterogeneous samples.
Eco-reagents
The sole aim of the extraction, synthesis, and sample preparation approach with eco-friendly functional material is to reduce and replace the application of deleterious chemical, costly and toxic materials. These green materials consume less energy, reduce overall reaction time, and minimize solvent waste in the sample preparation approach. Thus, enhancing and ensuring safety by providing environmentally responsible alternatives during chemical analysis, consequently making the entire analytical process green [31]. In recent years, the utilization of green solvents, otherwise known as ionic liquids, has been greeted with much-vested interest in research owing to their exceptional physicochemical properties. [32]. In sustainable development, one of the most important priorities is reducing and completely eradicating toxic, non-biodegradable organic solvents from non-renewable sources. The replacement of these toxic reagents and solvents with natural, novel, eco-friendly, green solvents such as Deep Eutectic Solvent (DES), supercritical fluids, and Ionic Liquids (IL), stimulates their increased application in diverse fields and analytical methods, also enhancing Occupational safety (PR 12).
However, these ionic liquids (IL) have some disadvantages, such as toxicity, corrosivity, non-biodegradability, non-biocompatibility, and costly synthesis process. The emergence of a new class of solvents called the Deep Eutectic Solvents (DESs) that are economical, eco-friendly, highly- biocompatible, non-toxic, biodegradable, adjustable viscosity, and simple in fabrication are much better when compared to the ILs. Abbott et al. introduced Deep eutectic solvents (DES) as a wide range of novel green liquid solvents with excellent toxicological and physicochemical characteristics [33]. The word “Eutectic” comes from the Greek "ευ" (eu means easy), and "Τήξις" (teksis means melting) is described as a mixture of a compound capable of forming a joint super lattice that can melt and simultaneously freezes at temperature (60 °C) lower than the melting points of their separate individual components. Abbott and co-researchers later introduced carboxylic acids such as citric acid, tricarballylic acid, malonic acid, phenylpropionic acid, oxalic acid, phenylacetic acid, and succinic acid as a constituent of DES [34]. Choi et al. in 2011, proposed that sugars (like fructose, trehalose, glucose, and sucrose), amino acids (e.g., proline), and organic acids (like maleic acid, citric acid, aconitic acids, and malic acids), which are primary metabolites from plant origin can be extracted and synthesized using DES [34,35]. These unique physicochemical properties are enhanced by the Melting point depressions resulting from the complex hydrogen bonding arising from the individual components in the DES Systems [36-38] The use of choline chloride-based eutectic mixtures in synthetic applications stand out in the DES family because of its inherent multifunctional potential, topological transformation, organizational properties in the development of advanced functional materials as well as overcoming constraints with traditional analytical processes (typical organic solvents) and procedures [39]. Other applications such as the synthesis of electropolishing [40,41], eco-friendly solvents extraction [42,43], carbon nanotubes [44,45] catalysis and electrochemistry [46], metal oxides dissolution [47], organic and enzymatic reactions, and dopamine sensor [48] are carried out < 60 °C. This designer solvent (DES) has garnered much attention from both academia and industrialist in a variety of applications over the years as a result of its unique, attractive physicochemical properties such as high thermal stability, low volatility, low melting point, high solubility, which can also be enhanced by tuning the individual reacting DES components [36]. Additional advantages of these DESs revolve around their ease of preparation. That is, they can be easily synthesized from a low-toxic natural material, which is affordable and available through thermal mixing or freeze-drying affordable [49]. DES is synthesized from solid compounds, which become liquid in proper molar ratio and at a specific combination. There are three different approaches to mixing DESs: (a) Thermal mixing on the hot plate at 80oC with vigorous continuous mixing using a magnetic stirrer for less than 2 h producing a clear colorless liquid. (b) By complete dispersal and dissolution in water before vacuum evaporation in a rotatory evaporator at 50oC and finally, (C) By freeze-drying dissolved components in an aqueous medium.
DESs are subdivided into 5 groups, namely:
Type I The general formula is Cat+X−zMClx, where X− is a Lewis base (x and z refer to the number of Cl− and MClx, respectively. The low melting point requires a small number of non-hydrated metal halides. Non-hydrated metal halides comprise the bulk of DESs, e.g. (MClx) and quaternary ammonium salt (Cat+X−).
Type II Hydrated metal halides comprise this class of DESs, e.g. (MClx.yH2O) and salts (y is the number of H2O molecules), as shown in Figure 3.
Type III combination of two or more of the following: choline chloride (ChCl) with HBDs like alcohols, amides, and carboxylic acids, as shown in Figure 3.
Type IV these DESs are formed from the mixture of HBDs with suitable metal halides. For example, Abbott et al. formed eutectic mixtures and reported the combination of ZnCl2 with a variety of appropriate HBDs, including ethylene glycol, urea, acetamide, and 1,6-hexandiol [50], and finally,
Type V This type of DESs is formed from the mixtures of non-ionic compounds to synthesize a new class of DES with decreased freezing points [51], as shown in Figure 3.
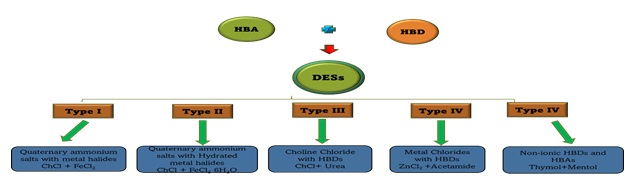
Figure 3. Subdivisions of DESs
Also, using supercritical fluids in place of traditional solvents as green alternatives for extraction techniques, such as subcritical water extraction (SWE) and supercritical fluid extraction (SFE) has gained a lot of application since its inception. This approach was first introduced for extraction by Hannay and Hogarth in 1897 but made public and pronounced by a renowned scientist Zosel for the decaffeination of coffee, for which he was credited with patency in 1964 [52]. This approach consists of a mobile phase CO2 tank, pressure-sized gas pump, oven that houses the extraction chamber, pump and co-solvent, and control valve with flowmeter attached to alter or maintain high pressure in the trapping vessel's entire system. This garnered much attention over the years from researchers in various fields of study, such as nutraceutical, polymer, pharmaceutical, and food industries, especially in the decaffeination of coffee [53]. The supercritical state is significant because substances, regardless of the state of matter, are subjected to pressure (Pc) and temperature (Tc) beyond their critical points and form supercritical fluids, where their specific properties and state vanish and can not be recovered with modifications in pressure or temperature. Also, supercritical CO2 is an alternative solvent which made the supercritical fluids a popular sample preparation technique, especially with the innovative capillary column supercritical fluid chromatography (SFC)[54,55]
Analytical Methodology
The greenness of any analytical methodology must be satisfactory to the figures of merits for any given analyte. In line with the 12 greenness principles of GAC, principle numbers (PR) 1, 3, 4, and 5 are the ideal greenness approach that can give comprehensive results from a one-step analysis approach (Direct analytical techniques (PR1), Automated system (PR3), Integration of analytical procedures and methodology (PR4) and miniaturization in analytical techniques (PR5)). The inculcation of microdevices allows the conglomeration of different steps, such as reactions, separation, and mixing, to coincide. Also, adopting and integrating adopting and integrating portable systems, remote sensing, and miniaturized systems make analytical methodologies green. Although each technique has its specific inadequacies and demerits, as shown in Table 3, the concern of the analytes should be directed toward the greenness of the approach as modified by different researchers [56-58].
Miniaturized Analytical Instrumentation
The Integration of a miniaturized analytical system started in 1990 and has since gained wide acceptability due to its numerous advantages, especially the utilization of minimal sample and reagent volumes [59,60], this is also in line with the 12 greenness principles of GAC, and it is denoted by PR2. However, it is vital to investigate, assess and improve the greenness of the applied techniques or methods [61-63] and consider the maintenance of the instrumentation and the large carbon footprint produced. Instrumentation automation and miniaturization (PR 3 and 5), as well as Integration of miniaturized procedures and methods (PR 4 and 5), will eradicate these and further yield a reduction in the amount of solvent consumed and waste generated and treated (PR7). Also, online purification of analytical waste and adoption of reuse, recycling, and regeneration strategies will assist in a long way and invariably enhance occupational safety (PR 12). These techniques include green electromigration, electrochemical, spectroscopic, and chromatographic techniques.
Analytical Wastes generated and treatment
Generally, the more analytical steps, the more the solvent and reagents consumed, and the more analytical waste generated. However, this depends on the analytical method. Therefore, there is a need to apply green methods that can generate little or no waste <50 mL or mg ([64]). Likewise, the reduction in the use of reagents reduces the generation of waste. Consequently, procedures that offer such opportunities should be exploited, while modification of existing procedures to meet the greenness criteria should be applied.
The proper treatment of any waste generated (PR7) harkens to GAC greenness principles, but also passivation, degradation, reuse, recycling, and regeneration of waste should be executed online.
Developed Metric System Tools
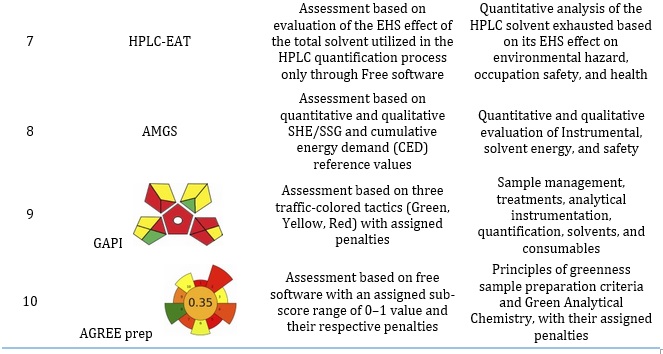
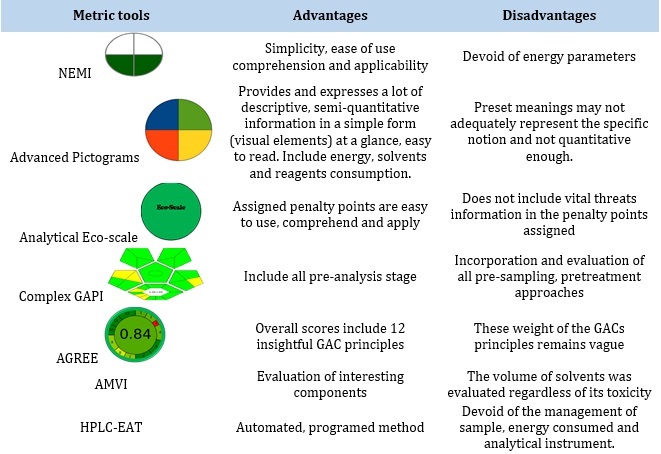
The first proposed metric approach was established, implemented, and reported in 2002 as the National Environmental Methods Index [3, 65,66] approach, which was based on parameters such as the toxicity of the product and process, corrosiveness, waste generated, and PBT nature of the solvent or reagent used (PBT: permanence, bioaccumulation, and toxicity). Subsequently, these parameters were modified by Garrigues and Guardia [67] [68] into a NEMI pictogram involving a grade scale of three colored traffic lights red, yellow, and green. The NEMI was later modified with the inclusion of energy consumption in 2009 by Raynie and Driver in 2009 into (the Green Assessment Profile) [69]. These parameters were investigated in dichotomy: the respective segment of the pictogram is filled green if any parameter value is met; otherwise, it remains colorless. However, another metric system was implemented, called the analytical Eco-Scale [10], which is based on allocating penalty points to each parameter that negatively impacts the greenness of the procedure in 2012. Parameters such as generated waste, the toxic nature of solvent /reagents, and high energy consumption were deducted points from base 100. In contrast, the remaining points after the user utilize deductions to evaluate whether the approach is satisfactorily green or sustainable. This approach evaluates the analytical approach using penalty points and the Eco-scale method [70]. Subsequently, another Innovative modification of the Eco-Scale was implemented, called the green certificate, based on new criteria. These criteria involved assigning weight penalty points and applying color code-related alphabets ranging from A to G, where A implies the most greenness criteria. Another approach was proposed and instituted in 2018 called the Green Analytical Procedure Index (GAPI), based on the NEMI, and Analytical Eco-Scale, which was utilized to evaluate the greenness properties of any analytical method from the sampling to final chromatographic or spectrometric determinations. It also employs a three-traffic light-colored code (green, yellow, and red) [3], as shown in Table 2. This multicriteria decision analysis (MCDA), such as RGB and Hexagon, enables the evaluation of the available analytical techniques using multiple assessment parameters such as eco-friendliness, the analytical performance of the method, and the economic impact simultaneously. It is also important to harness this multicriteria approach for all newly developed analytical procedures because each metric system supplies specific information about the method, including the pros and cons. Another metric system developed based on a Pictogram similar to NEMI is called Green Analytical Procedures Index (GAPI)[3], as shown in Table 2. The GAPI uses the pictogram to evaluate the greenness of each step (procedures, reagent/solvent, and instrumentation) in the analytical methodology, thus presenting the entire analytical protocol for the method. Although the GAPI used the same three-traffic-colored grade scale as the NEMI, however parameters evaluated in GAPI are much more than the NEMI approach [71], because each color in the three-traffic light color possesses another two or three levels of evaluation each, as shown in Table 2. The compactness of these GAPI pictograms allows for the ease of selection and comparison between the greenest method amongst others in the specified study. The GAPI also shows the weakest points in the analytical approach. This latest metric system is easy to interpret based on the RGB additive color model, comprising red, blue, and green colors, which have been interpreted as analytical performance, productivity, and greenness. The combination of these colors depends on the performance of each strata. The intensities of the additives of primary colors assigned to this methodology are also graduated on a scale of 0-100% with different distinctive ranges, thus simplifying the assessment of the RGB model and distinguishing the resultant final colors obtained. The method “Brilliance” is the qualitative parameter utilized for integrating the primary colors. The RGB model is evaluated using Excel worksheets. This newly developed model led to the formulation, innovation, and implementation of the white Analytical chemistry (WAC) concept [2]. The term “white” means a well-balanced, sustainable analytical procedure. The WAC allows the effective incorporation of the main assumptions of the GAC and other concerns without directly prioritizing the additional expectations over the integrity of the various parameters. The merits and demerits govern these metric systems. The major demerits of these metric systems are the addition of little non-continuous assessment and treatment parameters. Also, the earlier described metric systems did not evaluate all analytical procedures based on all the 12 principles of GAC. In addition, the results obtained do not relate to comprehensive information on toxicity structure or any other information on the analytical procedure evaluated. This review aims to develop user-friendly, comprehensive, and sensitive information on these innovative metric tools used to evaluate analytical procedures concerning the principles of GAC. In addition, beneficial importance and effects derived from the analytical data provided by these sustainable analytical metric systems are conveyed, increasing its awareness and potential to researchers in various fields. Also, Table 3 provides the advantages and disadvantages of greenness metric tools.
Table 2. Properties of Selected metric tools for the evaluation of greenness of an Analytical procedure

Investigating the five evaluation tools in an analytical procedure
The five blocks of evaluation tools investigated in any analytical procedure include figures of merits, sample treatment, characterization method, and calibration approach. For quality control and accuracy, figures of merits may also be applied to the toxicity nature of solvent and reagents, occupational risks, residue and waste generated, carbon footprints, and economic cost of analytical related to procedure and instrument damage calculations.
The Figures of merits
This shows the completeness and acceptability of the procedure concerning the sampling, sample collection, method, and quality control. The lower value obtained enhances the flexibility of the figures of merits to provide a definitive analytical result. This section also investigates the vital parameters contributing to the experiments and assigns them values (Penalty points (PPs)). Each parameter, especially the sample pretreatment, contributes towards the method’s fulfillment of the greenness GAC principles, such as no derivatization, preservation, transportation and storage conditions, micro amounts of sample, pretreatment, and dilution to required concentration for ease of characterization by the adopted techniques. In general, PPs may be or may not be assigned to the following depending on the metric tools used and time durations:
(i) for characteristics of the methods (in-line, off-line, and at-line methodology). (ii) Operational Mode (automation, semi-automation, and manual). (iii) Nature of sample/analyte (multi- or uni-component analytes). (IV) Method/sample (destructive and non-destructiveness) (v) portability, and (VI) robustness are either yes or no. Lastly, (Vii) time of analysis (< 10min< 100min ˃ 100 min). The PPs assigned to individual sections are between 0-4.
(2) Sample preparation and treatment: (i) Storage (physical, chemical, physicochemical, and none) (II) Preservation step (special, normal conditions or none) (iii) Amount of sample (Micro or macro). (vi) Number of reagents /solvents used (none, < 3 ˃ 3). (v) amount of solvent /sample mg(mL) ( (< 1(mL)(mg )(< 10(mL)(mg )< 50(mL)(mg) ˃ 50(mg)(ml)). (vi) instrumental adequacies (none, Dilute or concentrated, extent of dilution). (vii)Duration of weekly sampling (viii)Pretreatment method (none, filtration/drying and acid digestion). The PPs assigned to individual parameters are scaled from 0-4.
(3) calibration:(i) frequency (yearly, monthly, weekly, and daily). (ii) required time < 30min< 2hr< 8hr˃ ˃8hr). (III) Number of standards (< 5< 7 ˃ 7). (iv) R2 (R2< 0,99 R2˃ 0,99). (v) LOD and LOQ (vi) work linearity (suitable, not suitable). (vii) Precision (Horrat value (r/R) < Horwitz)( Horrat value (r/R) > Horwitz [5]). The PPs assigned to individual parameters are scaled from 0-4. It is worth noting that the precision is valued based on the repeatability of the results (Horrat); hence the RSDr is given as shown in Equation 1.
![]()
While the RSDR is evaluated as the calculated relative standard deviation based on reproducibility, C is the concentration of the target analyte in the sample.
Precision can be evaluated from the Horwitz equation, as shown in Equation 2.
![]()
Toxicity
The global harmonized system (SGA) [72] is the generally adopted method for the labeling and classification of various chemicals, solvents, and reagents, as shown in Fig. 4. Researchers have also evaluated these pictograms pasted on reagents/solvents for occupational and health hazards, assigning PPs to them [73]. The more the PPs assigned, the more the negative impact on health, safety, and the environment. On average, PPs evaluations for toxicity and safety hazards ranged from 22-24 PPs. The following parameters are evaluated based on physical, health, and environmental hazards. For health and environmental hazard, the criteria evaluated include corrosiveness, irritation (eyes and skin), carcinogens, and toxicity (aquatic and systemic poison to specific Organs). The PPs assigned to individual parameters are scaled from 0-4.
For physical and occupational hazards criteria evaluated are as follows: flammability, burns, spontaneous heat, corrosive for metals, presence of organic peroxides, pyrophorics, low-pressure gases, and self-reactive substances. The PPs assigned to individual parameters are scaled from 0-4. The overall quantitative assessment of all chemicals utilized in the analysis is evaluated from Equation 3, and the PPs assigned ranged from 1-3 scale.
![]()
Where A, B, C, and D are the amount of solvent or reagents utilized for sample treatment, total PP assigned to calibrations (frequent and Standard), calibrations (frequent and Standard) during quality control assessment, and frequency of validated accuracy, respectively.
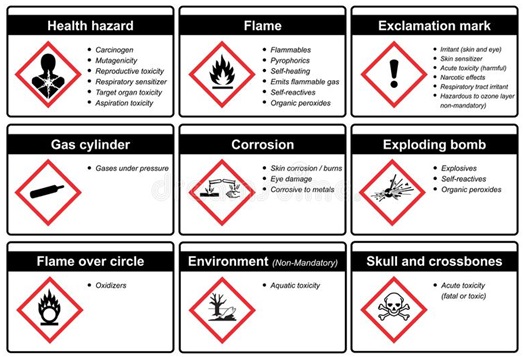 Figure 4. Pictograms of GHS for labeling and classification of chemicals
Figure 4. Pictograms of GHS for labeling and classification of chemicals
Waste generation
The greenness metric system aims to evaluate residue generated from any analytical methodology and assign penalty points depending on the amount of waste produced from such analysis. Also, the PPs are assigned to the likely means of waste treatment, waste processing, recycling, and disposal (plastic or glass medium) after the waste is spent or at the end of the analysis.
CO2 Footprint Evaluation
This is the criteria for evaluating and quantifying the impact of all analytical methodology on the environments, and it is expressed in the amount of CO2 Equivalent in Kilograms (Kg). The electricity consumed by any analytical instrumentation employed in the methodology must also be taken into consideration and analyzed based on time (in hours) concerning the constant emission factor value (0.247kg CO2/kWh)
Overall Cost
The yearly economic cost analysis evaluation is based on the criteria related to greenness GAC principles. These criteria include the annual average number of samples, time of analysis per sample, period of amortization, the salary of a skilled operator, electricity consumed, cost of reagents, equipment, and other consumables.
The relationship between the greenness principles of GAC, GSP approach, and 2030 SDG Goals
The interconnections between the GAC and the GSP principles are apparent and cannot be underestimated or treated sequentially. Although the GSP is not without its inadequacies, modifications, and redesigning can be made into GASCP for simplicity and greening of the sample preparation. Also, the ease of interactions between greening sample preparation procedures (GSP), GAC, and sustainable development goals 2030 established by the United Nations is not farfetched because GSP and GAC technologically contribute to the SDGs directly or indirectly, as shown in Figure 5. For instance, the GSP contributes to the physical well-being and good health of the operator and the environment by reducing the number of deleterious chemicals, reagents, and solvents released into the eco-space, thus making safer methodology which also reduces the occupational hazard around the operator, this complies with the SDG 3 and 8 as shown in fig.5. More also, the eradication of toxic chemicals, reagents and consumables, have positively impacted the quality of water, life below the water surfaces and life on land. This also is in line with SGD 6, 14, and 15. Modifying sample preparation protocols to sustainable sample preparation procedures also involve reducing modifying sample preparation protocols to sustainable sample preparation procedures involves reducing energy consumption and waste generation and adopting renewable/eco-friendly sorbents for recovery and extraction purposes. This is based on reuse and recycling methodologies which directly contribute to the reasonable production and responsible consumption of the SDG7 and 12 goals, as shown in Figure 5. SDG 7 postulated the consumption of clean and affordable energy using consumables from renewable sources. The efficient application of clean, cheap energy and maximizing size economy, including the eradication of waste, invariably contributes to sustainable societies, implying that SDG 11 is also in line with the combat of climate change SDG 13. Furthermore, reducing carbon footprint will alter the effect of greenhouse emissions, thus further cementing the National Climate plans proposed by the European Commission on July 14, 2021, with a target of reducing emissions by 55% in 2030.
The innovation and motivation behind implementing these metrics tools are to enhance technical, technological, and environmental benefits which are understandable, tangible, and more perceptible. This is the essence of assessing the greenness of analytical practices, especially sample preparation, optimizing and implementing it as a new sustainable analytical methodology to urgently address stressors and current global challenges critical to society and human and environmental health. Thus, empowering and enabling the greenness of GSP and GAC principles will contribute to sustainability.
 Figure 5. The interconnectivity between the greenness GSP, GAC principles, and the SDG 2030 goals
Figure 5. The interconnectivity between the greenness GSP, GAC principles, and the SDG 2030 goals
The developed key elements of the GSP are summarized
The developed critical elements of GSP are summarized and exemplified below, emphasizing salient characteristics necessary for greening sample preparation.
Portability of the method or in-situ sample preparation
This is a more sensitive and greener alternative that involves employing a device as all or part of the sample preparation (sampling or preconcentration) process in other to save time (PR 6) and energy (PR 8) and minimize the number of samples needed for the analytical processes, as such reducing the amount of generated waste (PR 4). It also aids miniaturizations (PR 5). It is crucial because, in most cases, samples are far from the laboratory. Scan be degradable in the transportation process, hence the need for permanent or temporary installation of the portable device on the sample, thus fully automating the sample preparation process [75]. In another way, it involves the Integration of both the sample collection and preparation steps or the use of a low, noninvasive, and non-lethal approach (in-vivo Sample prep.) in a rapid non-exhaustive, non-depletive extraction or a “free-form” (non-bound, free-concentration) separations of plant bioactive compounds as shown in Figure 2. Thus, eliminating micro- or other living organisms in the obtained sample matrices [76,77]. Such portable devices can include Sorptive tape extraction (STE), Direct Contact-Sorptive Tape Extraction (DC-STE) [78], SPME installations TF-SPME device [79], bio samplers [80-83]
The Use of Greener reagents and solvent
The critical aspect of all greenness sample preparation approaches is the nature of solvents and reagents. The complete replacement of toxic chemicals for solventless, or safer, greener reagents and solvents (IL, liquidities gases, surfactants, DES, bio-based, micellar solvent and Supercritical Fluids) [84-92] thus enhancing occupational, health and environmental health. This criterion is vital to reduced waste generation (PR 4) and Occupational hazard (PR 10). Also, the use of water as an extraction solvent in SWE (subcritical water extraction) [93]and other applications that allow the use of less concentrated reagents such as ultrasound energy, microwave-assisted UV digestion [100-102], wet digestion in a flow system [103-105], microwave-induced combustion [97-100], thus enhancing sustainability[94-96].
Adoption of reusable regenerated and renewable consumables
Adopting sustainable, reusable, regenerated consumables in the preparation of samples invariably reduces waste generated and occupational risk (PR 4 and PR 10). This also involves the synthesis and use of consumables for the microextraction (extraction) of the target analyte from complex environmental sample matrices. These consumables include sorbent based on Graphene and Graphene Oxide, molecularly imprinted polymers, and other nanoparticles in applications like fabric phase sorptive extraction [106-115]. These consumables can have novel potentials, such as improved mechanical and thermal stability, sorption capacity, reusability, and selectivity. Other advantages of these renewable bio-based consumables to Greenness GSP are low cost, biodegradability, reduced waste generation, and increased life cycle. Such materials include cotton, lignocellulosic materials, wooden tips, paper, polycarbonates, and aliphatic polyesters [4],[116,117],[121-130].
Zero-Waste Generated
This is another ambitious fundamental building block inextricably linked to AGREE metric system tool and greenness GSP, especially eco-benign methodologies. These approaches do not only offer the use of green, safer reagents, consumables, and greener non-reusable devices that are environmentally benign, such as glass tools and containers for extraction, recoveries, and digestions, but they also ensure the reduction and elimination of reagents or derivatization agents as well as operations and process consuming reagents and consumables PR (2 and 3). Also, the adoption of micro-based extraction operations (PR 5), suitable portable devices, and automation (PR 1 and 7) unvaryingly reduce the use of consumables and solvents, further minimizing operational and Occupational Hazards (PR 10). In addition, using alternative strategies like chemometric tools, specifically designed to reduce the number of experiments during the development and optimization of the method, is also valuable to greenness GSP. The design of the experiment approach includes Doehlert designs, central composite, Plackett-Burman, and Box-Behnken [131,132]. Finally, while ambitiously researching the zero-waste generated approach, it is paramount that administering proper waste management, treatment, and disposal system should be employed to enhance possible proper recycling of the consumables. Also, online decontamination, degradation, and Passivation processes should be adopted as waste management protocols [133-135].
Reduced number of consumables
The miniaturization step adopted in analytical operations towards greenness GSP reduces the generated waste (PR 4). When compared to the traditional approach, downscaling / reduced volume and size of consumables enhance the Zero-waste approach (PR 4) and has the propensity for automation (PR 1 and 7) and application of the portable device, thus yielding a robust, cheap, rapid procedures (PR 6) thus reducing the exposure of operational and occupational risk (PR 10) [85,136]. Also, there should be caution on reducing the sample size/volume so the potential representative sample will not lose its significance, thus jeopardizing/ deteriorating the analytical properties with enhancing the greenness of the method [137,138]. Summarily, sequential actions involving removal, replacing, reducing, regenerating, and recycling consumables based on the nature, size, and volume of the chemicals, solvents, and reagents should be engaged towards greenness GSP [139].
Enhanced Sample Throughput
The inclusion, treatment, and characterization of multivariant analyte in significant numbers/ volume sample matrices per time reduce the energy consumed (PR 8). It reduces analytical expenses and occupational and environmental hazards. To maximize this approach, dual treatment methods are employed viz a viz altering and accelerating the sample preparation steps to the analytical instrumental analysis (PR 9) and (ii) treating the various samples simultaneously, such as in 69-designed parallel electro membrane extraction [140,141], vortexing [142], the combination of various assisting fields [143] (ultrasound [144] or microwave and SPME (Eibak et al., 2014) [145] which can take longer preparation time but improves the overall turnaround time and reduces the energy consumed and accelerates extraction efficiencies in any micro-(extraction) techniques. The assisting field strategies accelerate mass transfer in sample preparations [146] by minimizing sample particle size (PR 5), adopting automated systems (PR 7), and lastly, enhancing phase transfer based on the Integration of additives towards partitioning, adsorption, chemical based conversion, and membrane sized- classification.
The Integration and adoption of the automation process
The preparation of samples consists of various stepwise procedures that are energy-sapping and time-consuming, which in the long run, can adversely alter the accuracy and precision of the method exclusively when handling complex environmental matrices. The Integration of operational steps like cleanup, derivatization, injection, and extraction [147-149] into sample preparation aims towards operational simplicity, reduces the consumption of chemicals, solvents, energy, consumables, risk contamination [150], and waste generation (PR 4,5,6, and 8). In addition, the adoption of automation processes (such as flow-based systems) enhances online sampling, increases sample throughput, and reduces human handling errors, occupational hazards, and artificial accidents (PR 10) [149,151-153].
Minimal Energy consumption
In the sample preparation procedure, the frequent adoption of heating systems (Soxhlet extraction) involving temperature gradients (elevated temperatures) in the presence of solvents for improving accelerating analytes transfers over a long period. This can be replaced with contemporary solventless heating extraction methodology, vacuum sampling for sampling involving headspace (micro)extraction or microwave heating system [154], and sonication [155]. Also, this reduces carbon footprints when energy from a renewable source is engaged for separation, purification, cleanup, self-separation, and isolation of extraction medium from complex environmental sample matrices by applying an external magnetic field [25].
Greenness analytical instrumentation and conditions
Using analytical instrumentation, such as green spectrophotometers, to characterize and analyze targeted multianalyte from environmental and food sample matrices requires low energy consumption and generates low waste (PR 4 and 8). Although the demerits of this analysis include poor selectivity, sensitivity, matrix effect, and poor reproducibility, some of the instrumental and analytical methodologies can be used to analyze final extracts from the sample matrices, such as capillary electrophoresis (CE), SBSE (stir-bar sorptive extraction), UVAE (ultrasound-assisted extraction)- HPLC and thermal desorption unit attached to the GC. Combining these techniques offers high extraction, recovery, separation efficiency, flexibility, rapid reaction time, and lower solvent consumption [4,90]. The greenness sample preparation methodology involves carefully selecting suitable instrumentation techniques, which are inseparably towards reducing toxic and hazardous chemicals, solvents, and consumables for specific analysis or characterization operations. It also saves energy and consumables and reduces occupational, health, and environmental hazards (PR8 and 10). The need and choice for analytical instrumentation depend on the analyst's interest, which can be based on accuracy, figures of merits, detection limits, or availability. It should also be toward the greenness of the applied technique.
Occupational Risk Reduced
The essence of this metric tool is to reduce the deleterious effect of sample preparation on the environment and analysts by minimizing the consumption or complete elimination of toxic solvents, reagents, and consumables. The danger of exposure and hazardous effect to/of toxic consumables as handling poses many risks to the operator and the environment. Hence, a multiapproach must be adopted to enable rapid, automated, and miniaturized sample preparations devoid of man-handing, thus minimizing occupational health hazards and exposure (PR, 5, 6, 7, and 9). Also, adopting solventless methods or safer solvents and consumables from renewable feedstock reduces exposure to occupational, health, and environmental hazard (PR 2, 3, and 4). Other occupational hazards that should be avoided include biological (diseases or hazardous allergens), physical, chemical, thermal, hazardous, irradiations, pressurized equipment, and compressed gases. Also, suitable protection and adequate storage system should be adopted for biological samples to avoid life-threatening emergencies during the GSP approach.
Computational Tools for Greenness Analytical Procedures
The utilization of computational kits in recent times has presented notable environmental and economic merits to enhance the choice of optimized processing factors or systems in analytical approaches, reducing the amount of energy/time consumed, amount of chemicals used, and waste generated. A variety of expert designs (experimental design) such as composite central Design (CCD), Box-Behnken in conjunction with RSM (Response Surface Methodology) are used to better comprehend and optimize the NADES/HDES synthesis conditions, extraction procedures (time, temperature, and solid/liquid ratio) and responses [90,156]. On the contrary, studying the variety of chemical moieties that can form HDES/NADES in any given experiment or application is experimentally impossible. This insilico method provides a medium of pre-screening approach for specific applications before exclusive experimentation, making it an interesting tool for selecting or designing suitable procedures. Researchers have developed and implemented COSMOS-RS to screen 126 NADES to strongly dissolve/solvate triolein, rutin, and trilinolein. The Conductive-like Screening Model for Real Solvents (COSMOS-RS) gave insight into the interactions of rutin with multi-constituent NADES systems that have never been practically studied or experimented [156,157].
Table 3. Summarized advantages and disadvantages of selected greenness metric tools

Conclusion and Future Prospect
Different strategies, modifications, and improvements are needed to consider analytical methods green and sustainable. These changes are essential to alter some or the whole process from the sampling to the treatment of generated waste while eliminating toxic consumables, elevated critical conditions, and adopting the zero-waste steps towards the greenness features. Integrating the GSP and the GAC principles is needed to formulate new universal green approaches required for future quantitative, green, renewable safer consumables and sustainable methodologies. This may be challenged by the diversity and requirements of these methods, experimental conditions, physicochemical properties of the reagents, target multianalyte, and complex sample matrices, notwithstanding, the roadmaps created and is sufficient to shade light for methodical advancement of Greener analytical in situ sample preparation chemistry, with emphasis on mitigating Operational, health and environmental hazards and creating more hotspot for research. Also, enhancing sample throughput is essential and paramount, as well as microparticle size sample economy, miniaturization, automation, and portability of the process towards sensitive, rapid analytical information. Also, figures of merits such as precision, sensitivity, robustness, selectivity, and accuracy should not be underestimated but optimized. These metric tools systems have also been integrated to enable and empower some of the 2030 sustainable development goals (SDGs). However, there are still a few Current global stressors left uncomplemented, hence another urgent multidisciplinary research hotspot.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by the Tertiary Education Trust, TETFUND Nigeria, and The Federal Polytechnic Offa, Kwara State, Nigeria. The authors sincerely appreciate this support.
Disclosure statement
The authors declare that they have no conflict of interest
Orcid
Aduloju E. Ibukun : 0009-0009-5468-6283
HOW TO CITE THIS ARTICLE
Emmanuel Ibukun Aduloju*, Noorfatimah Yahaya, Nadhirah Mohammad Zain, Mohammad Anuar Kamaruddin, Muhammed Ariffuddin Abd Hamid. A Green, Sustainable, and Unified Approaches towards Organic and Inorganic Analytes Extraction from Complex Environmental Matrices. Adv. J. Chem. A, 2023, 6(3), 198-224.
DOI: 10.22034/AJCA.2023.393229.1361


