Document Type : Original Research Article
Authors
1 Department of Chemical sciences, Bamidele Olumilua University of Education, Science and Technology, Ikere,- Ekiti, Ekiti State, Nigeria
2 Theoretical and Computational Chemistry unit, Department Chemical Sciences, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
Abstract
The Alchornea laxiflora leaves were introduced as a sustainable corrosion inhibitor with excellent inhibition action on mild steel in 1.0M acidic medium solution. Alchornea laxiflora leaves are found throughout Africa, including three active compounds: quercetin, Quercetin-3-rhamnoside, and Quercetin-7-rhamnoside. The LC-MS and NMR are utilized using the gravimetric method to track the active components in the Alchornea laxiflora leaves extract. Potentiodynamic polarization, electrochemical current noise measurements, and scanning electron microscope (SEM) were conducted to investigate the corrosion inhibition role of various concentrations of Alchornea laxiflora extract toward mild steel corrosion in 1.0M acidic medium solution. Surface analysis, molecular dynamics, and quantum mechanics simulation methods were combined to get insights into inhibitor molecules’ adsorption on the mild steel surface. The results showed that the inhibitory capacity of the leaf extract increased with an increase in the concentration of the extract, whereas the corrosion rate of the steel material after immersion in the acidic solution decreased with increasing extract concentration. The structures were determined using nuclear magnetic resonance in 1D and 2D, and the molecular weight was determined using mass spectrometry. The electrochemical investigations revealed that the maximum inhibition efficiency of 98.8% was obtained in the present extract. The formation of protecting layer on the mild steel surface was proved by scanning electron microscope and atomic force microscope results. The results derived from Monte Carlo simulation MC and QM calculations revealed the adsorption of Alchornea laxiflora components on the steel substrate via donor-acceptor interactions.
Graphical Abstract
Keywords
Main Subjects
Introduction
Corrosion is a prohibitive issue for a diversity of industries. Detection and discounting of the expenditure of metal corrosion have been of great interest to corrosion engineers and scientists for many decades and is still snowballing. Corrosion-resistant materials, corrosion inhibitors, anodic/cathodic protection, protecting coatings, corrosion inspection and monitoring tools have been fiercely used in abundant applications to protect metallic structures from corrosion and reduce their cost [1-4]. The popular and conventional corrosion protection strategy for the safekeeping of metals is the application of corrosion inhibitors. Despite the excellent inhibition activity of various inhibitors, most of them are not environmentally safe [5-7]. Corrosion inhibitors are defined as the ability of substances to effectively reduce the metal corrosion rate when added at low concentrations to harsh environments [8-10]. They can be categorized into two domains of organic and inorganic inhibitors [11-12] according to chemical structure. Recently, many investigations have dealt with developing the relationship between inorganic-organic corrosion inhibitors in various media [13-15]. However, the inhibitors primarily utilized in the industries are composed of some toxic compounds with many criticisms due to their threat to humans and the environment. Therefore, the use and production (industrial scale) of various sustainable inhibitors have become the topic of such research in recent years [16-19]. The modern context is to expand the eco-friendly and inexpensive corrosion inhibitors based on the components taken from the plant extracts as a readily renewable source. Several research groups have studied the significant inhibition performance of the sustainable corrosion inhibitors extracted from the plants for corrosion control of metals in acidic media [20-25]. The corrosion inhibition action of the Nettle leaves extract with inhibitive molecules like Quercetin, Histamine, Caffeic acid, and Serotonin has also been proven [28-30]. The inhibition power for Cichorium intybus L and Nettle leaves extract was 26% and 51%, respectively. Although there are some reports on the application of plant extracts as a powerful source of corrosion inhibitors for metals in various medium most of them are not readily available on a large scale and are cost-effective. Thus, using them in industrial applications would not be economically acceptable. Because of the above discussion, several factors must be considered when choosing an inhibitor for a specific application, including high inhibition efficiency, cost, easy availability, and most importantly, the environment's safety.[31] The inhibitory efficiency of these environmentally friendly inhibitors has been studied using several experimental approaches such as the gravimetric method [30], potentiodynamic polarization technique [32], hydrogen evaluation method [33], and energy dispersed spectroscopy (EDS) [30]. Based on the reason given above, Alchonea laxiflora leaves were chosen for this study. The leaves are a shrub that belongs to the Euphorbiaceae family and is found throughout Africa. In Nigeria and other regions of Africa, the leaves are commonly utilized in folk medicine. According to the literature studied, the plant possesses phytochemical elements [34,35] that can be employed as corrosion inhibitors. Furthermore, the materials are affordable, widely available, and environmentally friendly [31]
Materials and methods
Materials
Alchornea laxiflora was the plant material studied in this study. It was collected near Federal Polytechnic Ado - Ekiti in Ekiti State, Nigeria, and authenticated at the Department of Biological Sciences, Bamidele Olumilua University of Education, Science, and Technology in Ikere, Nigeria. Universal Steel Company, Ogba Lagos, Nigeria, supplied the mild steel for this study, and the chemical composition was analyzed at the same location. Chemicals for this study were obtained from Thermo Fisher Scientific Inc. HCl (38%), product No.351280-500, methanol, dichloromethane, ethyl acetate, and n-pentane. Corrosive solutions (electrolytes) were created by diluting concentrated acids (1.0 M) with deionized water to the desired concentration. All experiments were conducted in undisturbed solutions, and all measurements were taken with an analytical weighing scale (Metler Toledo PB153). A rotary evaporator, a Liquid Chromatography Mass Spectrophotometer, a Nuclear Magnetic Resonance, distilled water, a beaker, a measuring cylinder, a thermostatic water bath, paper tape, Whitman filter paper, and a desiccator were also utilized
Preparation of plant samples
Alchornea laxiflora leaves were cleaned and air-dried before being pulverized and sieved through an 850 μm sieve. The material was then extracted with methanol using the maceration method [36]. After three days, the mixture was squeezed by filtration through a 0.25 m filter, and the filtrate was placed in a thermostatic water bath at 600oC to get the air-dried Alchornea laxiflora leaves to concentrate. Before use, the dried extracts were reweighed and stored in a brown bottle at room temperature.
Mild steel preparation
The mild steel sheets were physically pressed and cut into coupons measuring 2.5 2.5 0.4 cm. A small 5 mm hole was drilled near the upper edge of the coupons to aid in their retention with glass hooks when suspended in the corrosive solution. The mild steel was polished with a struer polishing machine and various grades of emery paper before being rinsed with distilled water, dried with nitrogen gas, and stored in humid-free desiccators [37].
Corrosion inhibition Studies
The inhibitory efficiency of Alchornea laxiflora leafs methanol extract and the mild’s corrosion rate after immersion in the acidic solution were determined using a gravimetric experiment. A metal coupon of known weight was immersed entirely in 100 mL of 1.0 M HCl in the absence and presence of various concentrations of extracts (0.2, 0.4, 0.6, 0.8, and 1.0 g) for four hours using glass hooks. After four hours of immersion, the coupon was removed from the acidic solution, washed with distilled water to remove the corrosion product, rinsed in acetone before drying completely with nitrogen gas, and re-weighed. Using equations 1 and 2, the corrosion rate (g hr-1 cm-2) of mild steel in the absence and presence of the extract, as well as the inhibition efficiency (I.E%) of the inhibitors, were computed [38].

Where w is the weight loss in grams calculated from the difference in the mild steel's initial and final weights, A is the cross-sectional area in cm2, T is the exposure time in hours, and CR1 and CR2 are the corrosion rates of the mild steel strip coupons in the absence and presence of inhibitor, respectively.
Isolation
The crude methanol extract of the Alchornea laxiflora leaves was subjected to a purification process detailed below. The methanol extract was treated with dichloromethane and ethyl acetate successively. The dichloromethane and ethyl acetate extracts were filtered and dried on a rotary evaporator.
Liquid Chromatography Mass Spectrometry
The LC-MS/MS analysis was performed using an Agilent Technologies 1220 series LC system with an Agilent 6100 series quadrupole mass spectrometer in ESI/APCI mode. An Agilent Eclipse C18 4.6x50 mm column was used for separation; flow rate: 1 mL/min; detection: 254 nm; sample volume: 10 l; mobile phase: acetonitrile/ 5mM ammonium acetate: water/5mM ammonium acetate; 5%, 1.48 min; 5-100%, 8 min; 100%, 13.5 min; 100-5%, 16.5 min; 18 min. The LC-MS sample was generated by dissolving roughly 1 mg of the isolated chemical from Alchornea laxiflora leaves in a few drops of dimethyl sulfoxide [(CH3)2SO4] and then diluting the resultant solution with 1 mL of acetonitrile: water (1:1) prior to running.
Nuclear Magnetic Resonance (NMR) spectroscopy
The isolated sample was treated with n-pentane and allowed to stand before being prepared for NMR analysis. The extracts formed a greenish-colored precipitate.They were filtered and dissolved in deuterated chloroform (CDCl3); proton spectra were obtained with a Bruker AV3 500 MHz NMR spectrophotometer. NMR spectra were collected using a Bruker AV500HD (500 MHz) instrument and analyzed with Advanced Chemistry Development Labs' (ACD/labs) NMR processor 12.00 or MestReNova 10.0 software. Chemical shifts (d) are measured in parts per million (ppm) in comparison to an internal solvent reference (tetramethyl silane), and coupling constants (J) are measured in Hertz (Hz). Singlet (s), wide singlet (br. s), doublet (d), doublet of doublet (dd), triplet (t), quartet (q), and multiplet (m) were used to denote splitting patterns (m).
Scanning Electron Microscope / Energy Dispersive Spectrum
The surface morphology of the mild steel specimen before and after immersion in the acidic medium in the plant extract’s absence and presence was evaluated using a scanning electron microscope.
In the sample preparation, four mild steel samples were taken and polished to a mirror finished using 400 to 800 grit emery paper placed on a struer polishing machine, washed with distilled water, and dried using nitrogen gas before the initial weight of the samples was taken. The first and second mild steel was immersed in different 1.0M HCl containing 0.2 and 1.0 g/L of the methanol extract of Alchornea laxiflora leaves. The third sample was immersed in the 1.0M HCl without the leave extract, while the fourth sample was not immersed in either acidic solution. After 24h of immersion, the mild steel was removed from the acidic solutions and dried using nitrogen gas before the SEM image, and the EDX of the samples were taken using HITACHI SU6600 Scanning Electron Microscope.
Theoretical details
Ab initio QM optimization
Ab initio QM optimization was performed on the structure of some organic components comprising the Alchornea laxiflora corrosion inhibitor. The geometries of these compounds were optimized to find their lowest energy structures. For this purpose, all substances' potential energies were minimized using density functional theory in combination with B3LYP functional and 6-31G* basis set [39], as successfully used in our recent research [39,40]. The energy band gap, Eg, electron affinity, A, ionization potential, I, chemical hardness, η, softness, σ, electronegativity, χ, and the fraction of electron transferred, ∆N, were all calculated as earlier reported in the previous work [40]. The Spartan 16 program package was used for QM calculations. Subsequently, the HOMO-LUMO, partial atomic charges, and Fukui indices were calculated.
Monte Carlo simulation
Active compounds in the plant extract Alchornea laxiflora were investigated by Monte Carlo simulation with the aid of an adsorption locator module on Material Studio 2017 software using Fe (110) as a standard metal surface owing to plane miller indices [41-43] which suffice while searching for surfaces with well-packed structure and good stability [43]. The compounds quercetin, Quercetin-3-rhamnoside, and Quercetin-7-rhamnoside.were optimized with Forcite, while the surface of the metal was modeled (10x10 supercell; vacuum thickness = 30 Ȧ; box volume = 6). Five annealing cycles (50000 steps per cycle) were used to calculate fine-quality adsorption. The MC simulation on optimized low-energy Fe (110) was performed using water and HCl media to mimic a real-life setting. The molecules were adsorbed on Fe (110) surface using an adsorption locator module with a COMPASS forcefield. The adsorption energy was calculated [44].
Results and discussion
Effects of extract concentration on its inhibition efficiency
The effect of extract concentration on inhibition efficiency found that increasing extract concentration increased inhibition efficiency (Figure 1). This increase in concentration made more phytochemical elements of the extract available, which are then adsorbed onto the metal surfaces, causing the reaction sites to be blocked and safely protecting the mild steel surfaces from corrosion ions in the acidic media [45].
Effects of extract concentration on corrosion rate
Figure 2 depicts the effect of increasing the extract concentration on the corrosion rate of mild steel in 1.0M HCl. The results show that the corrosion rate of mild steel in acidic media decreases with increasing extract concentration as a result of an increase in the phytochemical constituents of the extract that are available, which are successively adsorbed on the mild steel surface, creating a barrier for charge and mass transfer, resulting in a reduction in the interaction between the metal surface and the corrosive medium, and thus decreasing the corrosion rate [46].
 Figure 1. Plot of inhibition efficiency of Alchornea laxiflora leaves extract against its concentrations
Figure 1. Plot of inhibition efficiency of Alchornea laxiflora leaves extract against its concentrations
 Figure 2. Plot of corrosion rate of mild steel in the absence and presence of different concentrations of Alchornea laxiflora leaves extract
Figure 2. Plot of corrosion rate of mild steel in the absence and presence of different concentrations of Alchornea laxiflora leaves extract
Mass Spectrometry via Liquid Chromatography
From the LC-MS results, three peaks were detected by UV which implies at least three different compounds are present in the sample (Figure 3). It is believed that these compounds are all related because they all possess the m/z 303 peaks which are believed to be derived from the plant flavonoid, Quercetin (Figure 4a), Molecular weight: 302.2380; Formula: C15H10O7 or the m/z 305 peaks which are believed to be from an oxidized analog of Quercetin (Figure 4b) Chemical Formula: C15H12O7, molecular weight: 304.2540.
The first peak (Figure. 5a and 5b) is a minor product with m/z 100 % 437.1, which is believed to be one of the isomers depicted in Figure S1a S1b. The main peak found (Figure S2a and S2b, S3a and S3b), is envisioned to be comprised of two compounds evident from the split in the peak and is believed to contain either the 3- or 7 rhamnosides of Quercetin (Figure S4a or S4b), m/z 100 % 448.4 for the molecular formula: C21H20O11, as well as one of the de-hydro isomers depicted as Figure. S5a or S5b, m/z100 % 432.4 [M+H]+, m/z, 100 % 432.4 [M-H]- for molecular formula C21H20O10(432.4). The second minor peak found (Figures S6a and S6b), is envisioned to also contain another de-hydro analogue (Figure S7a and S7b) with m/z 100 % 432.4 [M+H] +, m/z 100 % 432.4 [M-H]- for molecular formula C21H20O10(432.4).
 Figure 3. LC-MS report for isolated compound from the leaves of Alchornea laxiflora
Figure 3. LC-MS report for isolated compound from the leaves of Alchornea laxiflora
 Figure 4. (a) Quercetin(C15H10O7), Mw: 302.2380, (b) Oxidized(C15H12O7) analogue of quercetin, Mw: 304.2540
Figure 4. (a) Quercetin(C15H10O7), Mw: 302.2380, (b) Oxidized(C15H12O7) analogue of quercetin, Mw: 304.2540
 Figure 5. (a) Positive Mass Spectrum for first Peak: m/v ESI (%) 305.1 (50%) [M-sugar]+, 437.1 (20%)[M+H+], (b) Negative Mass Spectrum for first peak: m/v ESI (%) 303.1 (30%) [M-sugar]+, 435 (100%) [M-H.]
Figure 5. (a) Positive Mass Spectrum for first Peak: m/v ESI (%) 305.1 (50%) [M-sugar]+, 437.1 (20%)[M+H+], (b) Negative Mass Spectrum for first peak: m/v ESI (%) 303.1 (30%) [M-sugar]+, 435 (100%) [M-H.]
Nuclear Magnetic Resonance
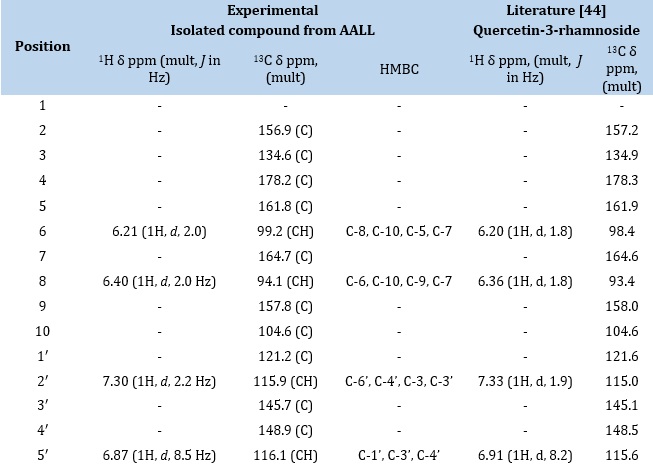
The proton spectrum (Figure 6a-c) showed an H-bonded hydroxyl proton at δH 12.66 ppm, typical of the 5-OH of a flavone moiety as shown in the general structure of flavones (Figure 7). It also showed two meta coupled aromatic protons at δH 6.21 (1H, d, J = 2.0 Hz, H-6) and 6.40 (1H, d, J = 2.0 Hz, H-8) on ring A while ring B had three protons in an ABX coupling pattern at 7.30 (1H, d, J = 2.2 Hz, H 2′), 6.87 (1H, d, J = 8.5 Hz, H-5′) and 7.25 (1H, dd, J = 2.2, 8.5 Hz, H-6′). The absence of a proton singlet expected for H-3 indicated substitution either by –OH, OCH3, or sugar, but the presence of an anomeric proton at 5.27 (1H, d, J = 1.9 Hz, H-1″) as well as other sugar protons at 3.99, 3.53, 3.23 and 3.17 ppm indicates the presence of a sugar moiety. The sugar moiety was confirmed to be a rhamnoside by a methyl doublet at 0.82 (3H, d, 6.0 Hz, H-6″). The 13C Dept-q Spectrum (Figure 8) showed 21 signals made up of one carbonyl at 178.2, an anomeric carbon at 102.3, five aromatic CH, ten quaternary aromatic carbons (seven phenolic, one carbonyl), and four oxymethine sugar carbons and one aliphatic methyl. Further examination of its 2D NMR spectra (Figure 9a-c) confirmed the attachment of the rhamnose sugar to position C-3 as a glycoside. The correlations and chemical shifts were typical of quercetin as the aglycone and rhamnose as the sugar. The mass spectrum confirmed the molecular mass to be 448.38 and the molecular formula as C21H20O11. The structure was further confirmed by comparison with literature Reports in Table 1, in which Quercetin-3- rhamoside was isolated from pometia piñata [46] and other reports in which quercertin analogs were isolated from the leaves of Alchornea laxiflora [34,47,48].
 Figure 6. (a) NMR spectra of isolated compound from methanol extract of AALL (b) Expanded proton spectrum of isolated compound from Alchornea laxiflora leaves extract (region 8.00-3.00 ppm) (c) Expanded proton spectrum of isolated compound from Alchornea laxiflora leaves extract (region 3.90-0.40 ppm)
Figure 6. (a) NMR spectra of isolated compound from methanol extract of AALL (b) Expanded proton spectrum of isolated compound from Alchornea laxiflora leaves extract (region 8.00-3.00 ppm) (c) Expanded proton spectrum of isolated compound from Alchornea laxiflora leaves extract (region 3.90-0.40 ppm)
 Figure 7. (a) Structure of flavones, (b) Structure of quercetin 3- rhamnoside
Figure 7. (a) Structure of flavones, (b) Structure of quercetin 3- rhamnoside
 Figure 8. 13CNMR spectrum of isolated compound from Alchornea laxiflora leaves extract
Figure 8. 13CNMR spectrum of isolated compound from Alchornea laxiflora leaves extract
Table 1. Comparison of NMR of isolated compound from Alchornea laxiflora leaves extract with Quercetin-3-rhamnoside isolated from pometia pinnata

Thermodynamic Studies
Thermodynamic properties such as Activation Energy (Ea), Enthalpy (ΔH°), and Entropy of Activation (ΔS°) are studied in order to identify the mechanism of the adsorption process involved. The activation energy for adsorption of the plant extract with varying concentrations at different temperatures was determined by plotting log corrosion rate (CR) Vs 1/T. From the slope, activation energy (Ea) was calculated from the formula.
Ea = - 2.303 x R x Slope
Where R is the gas constant (8.314J)
The data for Ea, involved in this study, are tabulated in Tables 2 and 3. From the table, it was revealed that the values of activation energy increase as the concentration of inhibitor increases. The value of Ea in blank solution under air dried samples of Alchornea laxiflora and Mucunna flagelleppes was 17.72kj/mol while that of the sun-dried sample was 12.50 kj/mol, and the values raise as the concentration of inhibitors increases from 0.2g/l (20.41, 19.95,19.89 and 17.87kJ/mol) to 1.0g/l (25.13, 25.88, 20.41 and 20.62 kJ/mol). This is due to the physical barrier created by adsorbed molecules on mild steel surface which increased the minimum energy required for corrosion reaction to occur, and the increase in the activation energy values with increasing concentration of the extract further corroborates the fact that inhibition efficiency increases with increasing concentration of the extract. In the present study, a physical adsorption mechanism is proposed since the values of Ea are lower than 80 kJ mol-1, and this happens due to the electrostatic force between a negatively charged metal surface and positively charged organic species. The trend of increasing Ea values as with concentration of the inhibitors have been reported by earlier studies on various plant extract such as jujube leaves, black pepper sunflower leaves, banana peels, and Alchornea laxiflora leaves.
Moreso, from the Errying Transition state plot, which involved the plotting of log𝐶𝑅/𝑇 vs 1/𝑇, the enthalpy change (ΔH°) for all the samples extract was obtained using the formula below.
Δ𝐻=𝑆𝑙𝑜𝑝𝑒𝑥𝑅, where R is the gas constant (8.314)
The enthalpy (ΔH°) values calculated for all the dried samples are positive, and it increases with an increase in the concentration of the inhibitor. The positive signs of the enthalpies reflect the endothermic nature of the mild steel dissolution process. The increase in the values of enthalpy change with an increase in concentration indicates that the addition of inhibitors retard the corrosion process, and more energy is needed for it to break the film barrier and react with a mild steel surface.
The entropy change (ΔS) values for the samples extract were also obtained from the errying transition state plot by using the formula:
Intercept = log (𝑅𝑁ℎ) + Δ𝑆𝑅
Which implies?
ΔS = Intercept – log (𝑅𝑁ℎ) X R X2.303 X 10-3
Where: h= plank constant (6.62617x10-34)
N = Avogadro’s Number (6.022045x1023)
R = gas constant (8.314)
From the table of the result, the values obtained for entropy (ΔS) change are negative for both air- dried and sun-dried Alchornea laxiflora and Mucunna flagellepes, which indicates that the activation complex in the rate-determining step represents an association rather than dissociation step.
Table 2. Values of Ea, ΔHoand ΔSoin the absence and presence of air-dried Alchornea laxiflora extracts in different concentrations

Table 3. Values of Ea, ΔHo and ΔSo in the absence and presence of sun dried Alchornea laxiflora leaves extracts in different concentrations

Scanning Electron Microscope
The SEM micrographs and the EDX analysis of the different slides of mild steel before and after immersion in 1.0 M HCl solution in the absence and presence of 0.2g/L and 1.0g/L of methanol extract of air-dried Alchornea laxiflora leaves was carried out by using HITACHI SU6600 Scanning Electron Microscope and the result obtained were shown in Figures 11, 12, 13. Here the spectrum exhibited a cocoon-like structure for a solution with the absence of the extract. This explains the surface was already undergoing a localized attack, which resulted in a cocoon-like structure, whereas there was an improvement in the surface morphology of mild steel treated with the methanol extract. The EDX analysis of each sample revealed that the uprising value of O is due to the formation of the ferrous hydroxide, while that of C is due to the presence of a phenolic compound called catechin, which acted as the active antioxidant and complexes with the mild steel surfaces.
More so, Fe, the major component of the mild steel sample, decreased after immersion in the acidic solution in the absence and presence of different concentrations of the extract (Table 4). From the result, it was observed that the fresh mild steel had the highest composition of Fe, followed by mild steel treated in 1.0g/L and 0.2g/L of the extract, and lastly, the mild steel immersed in the acidic solution without the plant extract. The EDX result also corroborates our findings that the corrosion rate of mild steel decreases with an increase in the concentration of the extract.
 Figure 10. SEM image and EDX spectra of fresh mild steel
Figure 10. SEM image and EDX spectra of fresh mild steel
 Figure 11. SEM image and EDX spectra of mild steel treated in 0.2g/L of alchornea laxiflora leave extract
Figure 11. SEM image and EDX spectra of mild steel treated in 0.2g/L of alchornea laxiflora leave extract
 Figure 12. SEM image and EDX spectra of mild steel treated in 1.0 g/L of Alchornea laxiflora leave extract
Figure 12. SEM image and EDX spectra of mild steel treated in 1.0 g/L of Alchornea laxiflora leave extract
 Figure 13. SEM image and EDX spectra of mild steel treated with 0 g/L of Alchornea laxiflora leave extract
Figure 13. SEM image and EDX spectra of mild steel treated with 0 g/L of Alchornea laxiflora leave extract
Table 4. The composition of the mild steel samples regarding the EDX analysis

Quantum chemical calculations
The energy band gap (Eg) values for Quercetin, Quercetin-3-rhamnoside, and Quercetin-7-rhamnoside in Table 5 are 3.67, 3.99 and 3.67eV (6-31G*) respectively, indicating that there is electron transfer from the molecule and the metal’s vacant d-orbitals, this is evidenced by the EHOMO value of -5.47, -5.79, -5.40 eV, indicating the excellent donating ability of the corrosion inhibitor, the LUMO orbitals have low ELUMO value of -1.80, -1.80 and -1.73 eV, indicating that the inhibitor can readily accept electrons from metal’s orbitals. The chemical hardness is a direct consequence of the Eg value, indicating that the principle of molecules, that is the hard and soft acids and bases (HSAB) hardness must be relatively low in order to enhance adsorption of the inhibitor molecule and the metal, the value of η is 1.84, 2.00 and 1.84 eV while δ is 0.54, 0.50 and 0.54 eV-1. When the metal’s hardness is assumed to be zero (very soft), it will react more readily with a soft base (the inhibitor molecule), thereby improving the adsorption and inhibition efficiency. To corroborate the molecule’s inhibited metal corrosion, the fraction of electrons transferred (ΔN) tells us how much the molecule binds to the metal surface. ΔN of an inhibitor must be < 3.6 for it to be considered effective [39]. ΔN for compounds is 0.83, 0.76, and 0.83, respectively, indicating that it has donating ability and is an effective corrosion inhibitor. The dipole moment is 4.00, 4.15, and 4.00, confirming that there is the distribution of electrons in the molecule and that there is a strong dipole-dipole interaction between Quercetin derivatives and the surface of the metal (that is, adsorption is enhanced through electronic force). The HOMO (Figure 14) shows-electron-rich molecular orbital is localized majorly on the benzylidene moiety, while the LUMO is delocalized almost entirely over the molecular system with major distribution over the phenol and azomethine moieties. This implies the possibility of intramolecular charge transfer in the system, as observed in our previous work [40]. The electrostatic potential maps (Figure 14) show-electron-rich benzylidene and hydroxyl moieties with different electron-deficient phenyl rings. The unsymmetrical charge distribution suggests a possibility of charge transfer between the benzylidene moiety and the phenol moiety.
 Figure 14. The optimized structure, the highest occupied Molecular Orbital (HOMO), the lowest unoccupied Molecular Orbital (LUMO), Electro potential Map of Quercetin, Quercetin-3-rhamnoside and Quercetin-7-rhamnoside
Figure 14. The optimized structure, the highest occupied Molecular Orbital (HOMO), the lowest unoccupied Molecular Orbital (LUMO), Electro potential Map of Quercetin, Quercetin-3-rhamnoside and Quercetin-7-rhamnoside
Table 5. The chemical parameters of the compounds at the DFT/B3LYP/6-31G(d) theory level

Table 6. The selected Calculated Mulliken atomic charges and Fukui functions of compound of Quercetin


Conclusions
The corrosion experiment demonstrated that the antioxidant activities of the extract increase as its concentration increases, resulting in a reduction in the corrosion rate of mild steel due to the adsorption of the organic compounds present in the extract on the mild steel surfaces. The analysis of LC-MS and NMR results indicated that the extract of Alchornea laxiflora leaves contains essential anticorrosive compounds. The separated chemicals may have contributed to the extract's inhibitory actions on mild steel in acidic media. These tests have demonstrated that the leaf extract has a good perspective and is an environmentally friendly organic inhibitor that can be used in medication development instead of synthetic inorganic inhibitors. The quercetin extracted from the methanol extract of AALL is an alternative corrosion inhibitor that can be used in industry for mild steel protection instead of the ecologically hazardous synthetic inorganic inhibitors
Acknowledgment
The authors acknowledge the Federal Government of Nigeria and the Research Laboratory of Prof. Sudipta Roy of the University of the Strathclyde, Glasgow, United Kingdom, for making funds and the available facilities, respectively.
Disclosure statement
The authors declare that they have no conflict of interest
Orcid
Oluwafemi L. Adebayo : 0000-0003-1648-9552
Timothy O. Esan : 0000-0001-6554-4947


