Document Type : Review Article
Authors
1 Department of Chemical Engineering, University of Mohaghegh Ardabili, Daneshgah Street, Ardabil, Iran
2 Department of Chemical Engineering, Faculty of Engineering, Ferdowsi University of Mashhad, Mashhad, Iran
Abstract
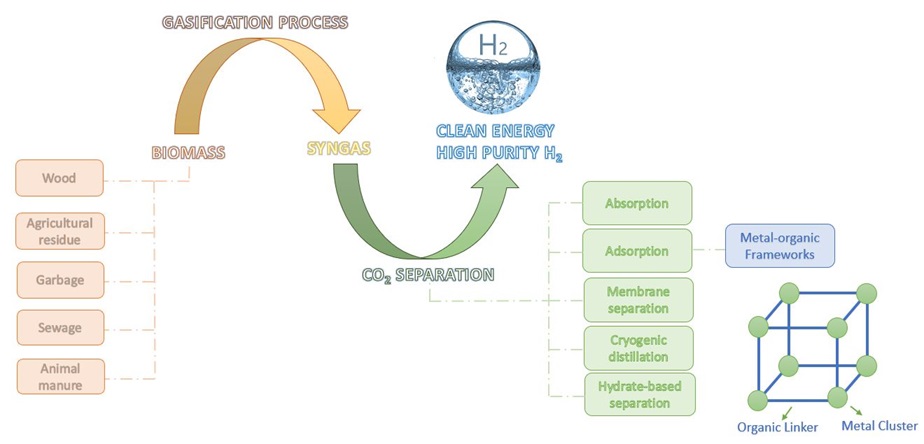
In recent years, considering the global warming and climate changes mainly resulting from greenhouse gas emissions, especially carbon dioxide, absorption and storage of carbon dioxide in generating clean and sustainable fuels such as hydrogen fuel production have been heavily studied and investigated. Researchers have presented various methods for carbon dioxide absorption in the hydrogen production process. The biomass gasification process, alongside absorption, can enhance the generation of hydrogen-rich gas by absorbing carbon dioxide. In this study, the first hydrogen generation in the biomass gasification process has been examined, followed by the technologies available for the absorption of carbon dioxide. This study reveals that developing novel materials for absorbing and separating carbon dioxide is essential. Given their unique physicochemical and structural features, metal-organic frameworks, including pore size, high thermal stability, high absorption capacity, and pore size tuning, are helpful adsorbents for absorbing carbon dioxide and achieving clean hydrogen energy. Thus, metal-organic frameworks (MOFs) may efficiently generate high-purity hydrogen by merging biomass gasification with CO2 adsorption.
Graphical Abstract
Keywords
Main Subjects
Table of Contents
Introduction
Carbon dioxide separation
CO2 adsorption using solid adsorbents
CO2 adsorption using metal-organic frameworks
CO2 adsorption in the biomass gasification process
Integration of the biomass gasification and CO2 adsorption
Conclusion
Introduction
Given the progressive growth of the population, energy demand has increased exponentially worldwide over recent years. This energy demand is one reason behind reducing fossil fuel reserves. In addition, fossil fuel usage is associated with environmental problems such as carbon dioxide emission, a significant greenhouse gas in global warming [1].
Studies show that human activities, especially the combustion of fossil fuels, are the main reason behind the elevation of carbon dioxide concentration in the Earth's atmosphere. These points indicate the importance of paying attention to reduce the adverse effects of the combustion of fossil fuels. To minimize the negative impacts of fossil fuel combustion, different scientific communities are trying to develop clean and renewable energy sources such as wind, biomass, and solar [1,2].
Biomass is a naturally occurring, renewable resource of biological origin readily accessible from several industrial sectors. Various forms of biomass, including forest and agricultural waste, livestock waste, urban waste, and fishery waste, such as sawdust, cellulose, straw, animal dung, and rice husk, are present in significant quantities [3,4]. Biomass energy has been considered sustainable because of its renewability, considerable reserves, and carbon neutrality. Concerning the reduction of fossil fuel reserves and environmental considerations, more attention has been paid to biomass for generating bioenergy [3,5]. Figure 1 displays the percentage of various renewable energies produced in the world. As observed in Figure 1, biomass has the highest percentage among various renewable energy sources such as wind, hydroelectric, solar, and geothermal.
Figure 1. The percentage of various renewable energies produced in the world [6].
Conversion of biomass to fuel or bioenergy occurs through biochemical and thermochemical methods. The biochemical conversion involves the hemicellulose degradation so that enzymes get access to cellulose, in which lignin remains unreacted and can be recovered and then used as a chemical or fuel in the subsequent thermochemical conversion process [7]. In all cases, the resulting fuel requires further processing to be usable without causing high engine corrosion, except for gasification, which leads to gas fuels without extra processing [8].
One of the most critical applications of biomass gasification is the generation of high-purity hydrogen [8]. Hydrogen is the cleanest alternative energy source, generating just water via burning and achieving "zero emission" of contaminants [9,10]. The heat value of hydrogen combustion is reported to be 142.3 MJ/kg, a figure that is three times more than that of petroleum [11]. This characteristic makes hydrogen a promising candidate for a clean, efficient, and sustainable energy source [12].
Figure 2 depicts main thermochemical processes to produce hydrogen from biomass. Several techniques have been proposed for producing hydrogen gas, such as water electrolysis, gasification with vapor, biological processes, and nuclear hydrogen generation. However, biomass gasification stands out among other technologies owing to its noteworthy attributes, including safety considerations, economic factors, and minimal environmental effects. The H2 production from biomass is often regarded as a highly efficient and cost-effective approach regarding both energy use and environmental consequences [13,14].
Figure 2. Main thermochemical processes to produce hydrogen from biomass [15].
Integrating the biomass gasification process with absorption can enhance the generation of H2-rich gas through CO2 adsorbing. Adsorption processes for purification and separation of gases are among the processes of purifying hydrogen from the syngas and removing acidic and greenhouse gases from the flue gas. Accordingly, economical adsorbents with high selectivity are used for adsorbing the material of interest and with relatively low adsorption heat [14].
In recent years, amine aqueous solutions such as monoethanolamine (MEA) and diethanolamine (DEA) have been widely utilized in carbon dioxide adsorption processes. Using these solvents in industries requires high recovery energy and staggering costs. Thus, developing novel materials for adsorption and carbon dioxide separation is essential [16].
Metal-organic frameworks are a new class of nanoporous adsorbents with great potential as adsorbents/catalysts because of their very high specific surface area, high adsorption capacity, orderly porous structures, and controlling pore size. Accordingly, they have different applications, including gas storage, separation, catalytic conversion, and carbon dioxide adsorption [17, 18].
The combination of biomass gasification with CO2 adsorption has garnered significant interest due to its potential to enhance hydrogen-rich gas production. In a study, the integration of these two processes was investigated in a double-fluidized bed system and compared with a process lacking the adsorption stage. The obtained results indicated that the purity of hydrogen generated in the integrated system was 75%, while only 40% in the adsorption-free system [19].
Recent advances regarding high-purity H2 generation from biomass gasification process have been investigated in this review. Initially, various carbon dioxide adsorption methods of various adsorbents that use metal-organic frameworks as CO2 adsorbents have been discussed. Furthermore, the studies conducted on the integration of biomass gasification process and carbon dioxide adsorption process have been examined.
High purity H2 using biomass gasification process
The gasification process is one of the thermochemical technologies for H2 production without emission of pollutant byproducts. The conditions of this process are more convenient over combustion and liquification. In this process, clean hydrogen energy can be generated efficiently and on a large scale from several types of biomasses. Therefore, biomass gasification is one of the most economical processes of H2 generation [20,21]. The conventional reactions in the biomass gasification process are presented as follows [22]:
Hydrogen generation from biomass has been studied through two thermochemical processes including:
(1) Conventional pyrolysis followed by modification of the carbohydrate part of the biofuel
(2) Gasification followed by modification of the syngas (H2+CO) [23].
The maximum obtained efficiency of hydrogen generation has been 46% and 55% (dry and ash-free volumetric percentage) from pyrolysis and vapor gasification processes, respectively. The efficiency of H2 generation in vapor gasification grows with an increase in the water-to-sample ratio and gasification temperature [20].
Biomass gasification is a multifaceted thermochemical process that exhibits high sensitivity to many process and operational parameters, including temperature, pressure, gasifying agents, equivalent ratio, and retention time. A slight change in process conditions might affect system performance and product quality [24].
The impact of temperature
The control of biomass gasification temperature is a pivotal determinant that profoundly impacts several facets of the procedure, encompassing gas composition, tar concentration, ash generation, gas composition, and reaction velocity. Therefore, regulating the temperature inside the reactor or gasification zone is essential to get the intended process characteristics. A high operational temperature is suitable for achieving a high carbon conversion rate, eventually lowering the tar content and generating more combustible gases. Nevertheless, it has been reported that the hydrogen concentration first grows, and then falls with temperature elevation [25].
The necessity of high-temperature gasification has also been reported for generating syngas with a high hydrogen yield in gasification with an air-vapor process in a bubbled fluidized bed (BFB) system [26].
Contradictory results regarding the temperature effect on the hydrogen yield have been reported, which can be due to differences in the examined biomass properties across various relevant studies. A study explored the effect of particle size within the range of 0.5-5 mm and gasification temperature within the range of 700-900 °C on the hydrogen yield. It has been observed that a considerable increase in the hydrogen yield would be achieved with a reduction of particle size and elevation of reactor temperature [27].
The effect of pressure
Generally, gasification is done under atmospheric conditions or at high pressures. The manipulation of biomass gasification temperature is a crucial factor that significantly influences several aspects of the process, including reaction rate, gas composition, ash formation, gas composition, and tar concentration. In some studies, a considerable reduction in tar yield has been reported with elevation of the gasifier pressure. On the other hand, other studies on the gasification of the fluidized bed have noted an elevation of the tar concentration with the increase in the gasifying pressure from 0.1 to 0.5 MPa. According to reports, a decrease in high pressure inside the gasification substrate has resulted in the generation of significant amounts of ash and a decrease in syngas quality [28].
The effect of gasifying agents
To meet the necessary conditions in subsequent processes, a range of gasifying agents, including air/oxygen, CO2, and vapor, are used to initiate the gasification process. The use of air as a gasifying agent results in the syngas production with a comparatively low heat value. The reason for this phenomenon is the gas dilution by nitrogen, resulting in syngas production with a moderate heat value when vapor and oxygen are mixed. The use of vapor in combination with air leads to a notable enhancement in the yield of hydrogen generation and a simultaneous decrease in the energy requirement of the system. In the water-gas shift reaction, vaporized air was employed to raise syngas H2 concentration. The process's thermal efficiency decreased with vapor. Pure oxygen as a gasifying agent is often considered advantageous in producing superior-quality syngas. This approach yields syngas with elevated concentrations of hydrogen (H2) and carbon monoxide (CO) while effectively minimizing the tar presence. However, using pure oxygen incurs significant costs, substantially impacting the system's economic viability. Using carbon dioxide as a gasification agent is linked to an increased concentration of carbon monoxide and a high calorific value of the resulting gas. Nevertheless, the chemical reactions associated with this process may need an extended duration for completion, resulting in a sluggish rate of reaction advancement [28].
The effect of equivalent ratio
Biomass gasification needs low extra air compared to complete oxidation, i.e. stoichiometric conditions, and the air (oxygen) to biomass ratio should be adjusted for the gasification. Accordingly, a parameter called equivalent ratio is considered. As mentioned above, the ratio is established as the quotient obtained by dividing the actual air content necessary for gasification by the stoichiometric air requirement for burning. The equivalent ratio significantly affects the gasification process and composition of the end product. A higher equivalent ratio leads to a decrease in the heat value of syngas. The literature suggests that an equivalent ratio of 0.2 to 0.4 has been identified as the ideal number for achieving efficient gasification [29].
The rise in the equivalent ratio leads to an increase in the methane concentration and a decline in the hydrogen concentration. This ratio for three types of biomasses, including wood, bamboo, and ipomoea, has been examined within the range of 0.24-0.27. Hence, the determination of the equivalent ratio is contingent upon the specific biomass feedstock used in the gasification process [30].
The impact of residency time
Concerning residence or retention time is crucial in designing a gasification reactor since it significantly impacts tar formation and gas composition. An increase in retention duration is associated with decreased oxygen-containing molecules and one and two aromatic compounds. However, it was shown that the presence of three- and four-ring tar compounds increased retention time. Moreover, the tar content has been reduced due to increased retention time in the biomass gasification with dolomite substrate [31].
Coal gasification in a bubble fluid system using oxygen and air as the gaseous medium at 875-975 ˚C and coal retention time of 15-55 min has been studied. The study revealed a positive correlation between coal particles' temperature and retention duration inside the gasification reactor and the carbon conversion rate. However, elevated temperatures and extended retention periods might lead to significant challenges in ash melting and the subsequent production of slag inside the reactor [32].
Therefore, it can be inferred that the gasification process is influenced by several variables, such as pressure, the composition of the feedstock, gasifying agents, equivalent ratio, and retention time, rather than only controlled by the operating temperature. Hence, optimizing all factors while designing an efficient gas delivery system is essential.
Carbon dioxide separation
Carbon capture technologies
There are three leading technologies for carbon capture, including precombustion, post-combustion, and combustion with oxyfuel, as illustrates in Figure 3. The type of fuel used, CO2 concentration, and the flow pressure that should be treated are the essential criteria for choosing the most durable and efficient technology for carbon capture [33].
Three main carbon capture methods are precombustion, post-combustion, and oxyfuel combustion. The most durable and efficient carbon capture system depends on fuel type, CO2 concentration, and flow pressure [33]. Post-combustion is effective for low CO2 concentration flows, whereas precombustion is used for high CO2 concentration flows [34]. Due to install post-combustion systems in power plants, the post-combustion system is more straightforward. Pre-combustion and oxyfuel systems need special modifications and can only be installed in new power plants [35]. These methods help biomass-based power plants provide energy without any modification.
Pre-combustion
The precombustion process is characterized by removing CO2 before gas combustion, as demonstrated in Figure 3. As mentioned above, the removal system is suitable for treating flows with high carbon content, often exceeding 20% of the total volume. One notable characteristic of this system is its ability to initiate a conversion process wherein fuel is initially transformed into a gaseous state by the controlled introduction of a certain amount of oxygen, air, or vapor. Gasification is a chemical process distinguished by the occurrence of partial oxidation, resulting in the predominant production of H2 and CO. The reaction products and vapor are added to a catalytic reactor for the water-gas shift reaction. This chemical reaction increases CO2 and H2, as the following Equations (10-12) [34]:
At this point, CO2 is absorbed, and H2 may power gas turbines, hybrid cycles, fuel cells, and gas tanks [29]. Natural gas and biomass could have been changed to produce syngas, and the water-gas shift reaction can increase H2 production. The endothermic vapor reformation process converts CH4 into H2 and CO at temperatures between 700-850 ˚C. An exothermic partial oxidation process converts CH4 to CO2 according to Equation (13) and Equation (14) [34]:
Post-combustion
As shown in Figure 3, the post-combustion system capturing carbon after syngas combustion and before greenhouse gas emissions in the atmosphere. This method is used in many natural gas power plants [26]. While other carbon removal systems need setup or initial settings, this technology may be quickly integrated with power plants. In the final step of the processing system, this technology allows process parameters to be changed without stopping power plant operations [36]. This process is helpful for gas power plants and those based on combustion that operate with biomass or coal.
Oxyfuel combustion
In this system, an air separation unit separates N2 from O2 before combustion. Next, oxygen and fuel enter an oxygen fuel tank to generate syngas, which only consist of water and carbon due to previous nitrogen separation, as illustrated in Figure 3.
The key benefit of this method is that it separates CO2 from synthase gas from water without desulfurization or NOx removal [30]. High flue gas CO2 concentrations range from 80-90%, depending on fuel [37]. The separated water is compressed and returned to the tank. The tank recovers flue gas to maintain operating temperatures. This method still faces hurdles, mainly linked to oxygen separation's high energy needs [34]. The air separation unit may raise energy costs by 7% compared to a power station without a carbon capture system [37].
CO2 separation methods
Some CO2 separation technologies tested in various research studies have proven effective in small or large power plants. The CO2 separation technique relies on power plant economic expenses, flue gas condition, and composition and operational characteristics. The practical approaches for CO2 separation are presented in Figure 4.
Absorption
The absorption process is often regarded as the most advanced technique for removing CO2. Absorption may occur using solid, liquid, or hybrid solvents. In the context of physical absorption, using a physical solvent, such as dimethyl ether polyethylene glycol (DPEG), facilitates the absorption of CO2 while selectively excluding other gases owing to their relatively lower solubility in contrast to CO2. In contrast to solid absorption, liquid absorption is considered as a more cost-effective option for flows with low concentrations of CO2 while also demonstrating reduced expenses for flows with high concentrations of CO2 [40].
Figure 3. Carbon capture technologies [38].
Figure 4. Practical approaches for CO2 separation [39].
A solvent can absorb carbon dioxide via a process known as liquid absorption, and then remove it through a series of chemical reactions. Subsequently, the solvent may be restored using pressure reduction or heating processes, contingent upon the specific solvent used, enabling its further reuse. The primary solvents used in this study are potassium carbonate, diethanolamine (DEA), diglycolamine (DGA), methyl diethanolamine (MDEA), and monoethanolamine (MEA) [37,41].
The reactions of DEA or MEA with CO2 are distinguished by their rapid reaction rates. Initially, a zwitterion is generated, which then interacts with CO2, forming a proton as shown in Equation (15). Then, a chemical reaction occurs between the compound above and an amine, resulting in the production of carbonate as shown by Equation (16). This carbonate compound then undergoes a reaction with water, leading to the bicarbonate formation and the liberation of free amine, as represented by Equation (17). Compared to primary or secondary amines, MDEA and tertiary amines have a considerably lower reaction rate with CO2, as shown by Equation (18) [42].
MEA is the most efficient amine-based solvent, removing over 90% of CO2 [37]. An aqueous solution with 20–30 wt% MEA is utilized. The fundamental problem of MEA is compressed energy needs during solvent production. An empirical investigation showed that incorporating a carbon absorption mechanism using MEA-based chemical absorption through a coal-based power plant may lower energy costs by 80% [41].
Amine-based solvents can produce technical and environmental difficulties due to solvent degradation with temperature increases, including reduced regenerated solvent and toxic emissions for environmental and equipment corrosion. Mineral-based absorption can fix these difficulties. Potassium carbonate has been researched as a competitive replacement for amine-based solvents. Piperazine chemical absorption increases potassium carbonate in a biomass-based power plant and energy efficiency by 43.8% [43].
Adsorption
Adsorption refers to the phenomenon where CO2 molecules attach themselves to the surface of a solid material known as an adsorbent. Commonly used adsorbents for CO2 adsorption include activated carbon, calcium oxides, hydrotalcite, lithium zirconate, and zeolite [37]. Adsorption is the process by which a material adheres to a surface. The selection of an ideal adsorbent depends on various factors, mainly related to the volume of removed CO2, kinetics, size of adsorbent pores, and structure. Zeolite is the primary physical adsorbent often used because of its intricate porous structure [34]. Next, CO2 immobilized on solid adsorbent can be removed using desorption techniques via temperature or pressure fluctuations, following which temperature or pressure grows after adsorption for CO2 elimination [37].
Calcium carbonate, a cost-effective adsorbent, has been extensively investigated for its use in both in-situ and ex-situ processes in gasification [44,45]. One of the primary benefits of using this approach is the commendable stability and durability, notable adsorption capacity, the minimal energy demand for the adsorbent regeneration, and notable adsorption capacity shown by the adsorbent [36].
Membrane separation
Selective semi-permeable membranes have the potential to be used in the separation of carbon dioxide from various fluid streams. The fundamental idea behind this technique is the use of selective semi-permeable membranes to filter one or more compounds, forming a distinct permeation. The membranes used in this context may exhibit organic composition, inorganic composition, or polymer materials encompassing metal, zeolite, carbon, or ceramic components. Their selectivity and permeability characteristics determine the choice of these membranes. Membrane separation has distinct benefits compared to other separation techniques, such as adsorption and absorption. Notably, membrane separation obviates the need for energy-intensive regeneration processes and may be seamlessly integrated with other systems [33].
One of the concerns associated with this approach is the flue gas condition that necessitates treatment. Low concentrations of CO2 and low pressure are undesirable as they can diminish the effectiveness of the carbon removal system [37].
Cryogenic distillation
The cryogenic distillation technique separates gases from other components based on their phase change behavior disparity. The process induces a phase transition of the material, converting it into a solid or liquid form and subsequent removal from the mixture. This approach necessitates the utilization of a multi-component phase diagram of CO2 and including additional chemicals present within the gas for the treatment [46].
Concerning its operational purpose, the system initially facilitates the cooling of flue gas, followed by further cooling achieved by using a heat exchanger. Subsequently, the gaseous mixture is directed towards a distillation column, including many trays or stages, where CO2 is extracted from the lowest level of the column [47].
The separation method described is well-suited for treating flue gases under high pressures. It can be effectively used in both pre-combustion and oxyfuel combustion systems. Furthermore, it may be used in the gasification process, whereby biomass serves as the primary input. Cryogenic distillation has shown significant promise in the context of liquid CO2 production objectives [46].
This technique also absorbs CO2 more efficiently than others. [47]. Nevertheless, one of the primary difficulties associated with this separation technique is the entity of water and CO2, which may lead to significant hindrances and impede the CO2 freezing. Accordingly, water should be eliminated before the cold stage, necessitating massive energy consumption [46].
Incorporating this segregation methodology into a power plant can result in a 50% augmentation in the overall operating expenditures. Advances in this separation technology are constant, with a vision toward proposing new cooling systems that resolve the issue of water obstruction and improve the system and energy efficiency [47].
Hydrate-based separation
Hydrate-based separation exposes flue gas to high-pressure water, where CO2 forms hydrates more quickly than other gases owing to their phase equilibrium. This separation process uses 0.57 kWh/kg of adsorbed CO2, a significant benefit [48].
The use of this system is more prevalent in pre-combustion applications than post-combustion technologies due to the more elevated levels of CO2 concentration and gas pressure, which facilitate the formation of hydrates. On the other hand, the exhaust gas produced by the post-combustion technique would require further processing, including compression, resulting in increased expenses [49].
In addition, this separation offers several environmental, technical, and economic benefits. The materials employed in this system are both non-hazardous and non-toxic. Furthermore, the system possesses a substantial storage capacity. It can efficiently absorb sulfur-based components without necessitating the implementation of supplementary processes or desulfurization. Additionally, the system facilitates the CO2 collection at high pressure, thereby obviating the need for subsequent compression prior to storage or separation, along with the associated expenses [48,49].
CO2 adsorption using solid adsorbents
To assess the efficacy of solid materials in separating CO2 from flue gases, it is essential to consider specific key performance parameters [50]. The factors included in this category consist of chemical, thermal, and physical stability, adsorption/desorption kinetics, adsorption capacity, adsorption enthalpy, cost of adsorbents, and selectivity.
- Adsorption capacity
The evaluation of solid adsorbent performance is contingent upon pivotal criteria. The provided data presents information on the capacity of a particular solid substance to sequester CO2. The relationship between the quantity of adsorbed CO2 and the mass of the adsorbent may be shown using weight adsorption (i.e. CO2 amount in gr/adsorbent gram or CO2 amount in cm3/adsorbent gr). Volumetric adsorption is another criterion for capacity, which reports the extent of CO2 adsorption in each adsorbate unit. This criterion is essential since it reveals the amount of the adsorbent required for a particular task and, thus, the size of the adsorbent substrate. It is also crucial in influencing regeneration energy [51,52].
- Selectivity for CO2
This parameter denotes the proportion of carbon CO2 adsorption relative to the adsorption of other gases, notably nitrogen in the context of post-combustion processes and methane in the case of natural gas. The assessment criteria in question have significant importance as they directly impact the purity of the adsorbed gas, exerting a substantial effect on the process of CO2 adsorption. One of the most straightforward approaches to estimating the selectivity coefficient is using single-component adsorption isotherms of CO2 and nitrogen [53].
- Adsorption enthalpy
It is another essential solid adsorbent performance parameter. It determines the energy needed to desorb the solid adsorbent, significantly affecting desorption cost, which measures CO2 adsorption strength and inclination.
- Physical, thermal, and chemical stability
To achieve cost reduction, solid adsorbents must demonstrate repeated stability cycles when subjected to flue gas exposure and adsorption operations across the whole of adsorption-desorption process. The solid adsorbent's sustained performance relies on its capacity to maintain stability in the presence of water vapor. The thermal characteristics of the solid adsorbent play a crucial role in heat transfer processes, alongside the thermal conductivity and heat capacity [53].
- Adsorption/desorption kinetics
The duration of the adsorption-desorption cycle is mainly influenced by the kinetics of the process, which experimental measurements can quantify. Adsorbents that can efficiently absorb and desorb CO2 within a shorter timeframe are considered more favorable since this is the reduced cost of separating CO2, the amount of absorbent needed, and cuts down on the cycle [52].
- Cost of adsorbents
The selection of adsorbents is a crucial consideration. Researchers primarily focus on materials that exhibit exceptional adsorption characteristics and are readily accessible at a minimal cost for CO2 absorption. Furthermore, synthesizing these materials poses significant environmental challenges that must be addressed [52].
Solid adsorbents used for CO2 absorption
Zeolites
Zeolites are porous materials composed of crystalline alumino-silicates that may be found in nature or synthesized artificially. The zeolite framework comprises tetrahedral T atoms, where T represents either Si or Al, joined by oxygen atoms. This arrangement forms rings with diverse pore sizes and shapes. The range of pore sizes inside the zeolite framework is reported to be between 5 and 12 Å [50]. Catalysts find extensive use in the refinery sector [55], chemical synthesis processes, and gas separation applications. Zeolites have been identified as very prospective materials for CO2 adsorption [56,57].
CO2 can undergo adsorption via many mechanisms, including the molecular sieving effect, which is based on the disparities in size seen in zeolites [54]. The separation process may also be achieved by polarization interactions between gas molecules and the electric field on charged cations inside the zeolite structure. Zeolites can regulate CO2 removal by modifying the framework's cationic composition, polarity, and pore size [59].
Among the different zeolites investigated for CO2 adsorption, zeolite 13X has emerged as the most extensively explored. It is generally considered the benchmark technology for solid adsorbents [59,60].
Research on using zeolites as an absorbent for CO2 absorption can be classified based on the approaches and techniques adopted for improving adsorption performance in various domains. These groups include adjustment of pore size, design of zeolites with controlled polarities, investigation of new zeolites, optimization of cationic exchange, and recently, combination of amine groups with other chemical functions within the zeolite framework [60].
Activated carbon
Activated carbon refers to a group of carbon materials with a high inner surface area and large porosity. It is used in different industries such as water and gas treatment. It can be prepared from various industrial products, wood, coal, and other biomass sources. Since activated carbon is synthesized from various materials, the final product has a diverse variety. Each product's features differ from others, thereby complicating the detection of this adsorbent [61].
The advantages of activated carbon for CO2 adsorption include high thermal stability, desirable adsorption kinetics, high adsorption capacity, large surface area, and suitable porosity. The downsides of activated carbon for CO2 adsorption include low adsorption capacity in the presence of interfering gases, such as hydrogen sulfide and nitrogen dioxide, reducing efficiency. Also, this adsorbent is sensitive to humidity, and its activity diminishes when exposed [41].
Graphene
Graphene is a thin surface of carbon atoms, developed in two dimensions and discovered in 2004 [58]. The first report related to CO2 adsorption using graphene was published in 2011. Concerning the high specific surface area and relatively low synthesis cost, today, graphene is of great interest to researchers for the adsorption and separation of greenhouse gases [63].
The significant features of graphene, offering a great role in CO2 adsorption, include the extent of synthesized graphene porosity, the use of the edge and surface of graphene for enhancing the absorption capacity, and augmentation of the adsorption efficiency based on functionalizing the prepared graphene [64].
Carbon nanotubes
CNTs are a type of carbon nanomaterials with a tubular structure. Carbon nanotubes consist of carbon atoms with sp2 hybridization. They have an integrated structure with honeycomb hexahedral lattices, several nanometers in diameter, and several micrometers in length. Nanotubes have unique features such as orderly pores, large pore volume, low density, and high surface area [65].
Due to good physiochemical features and high stability, these adsorbents have great potential for CO2 adsorption from the flue gas [66]. Nevertheless, the complex synthesis of this nanostructure and the relatively high cost of using this material for CO2 adsorption are limiting compared to other adsorbents.
Metal-organic frameworks
In recent years, there has been an emergence of a unique category of crystalline porous materials. The collection of components in question is often referred to as MOFs. MOFs are composed of metal-containing groups that are coupled by organic ligand bridges. These frameworks have primarily been constructed using robust, coordinated bonds. MOFs have a well-defined crystalline structure characterized by a three-dimensional porous network. This intricate architecture allows for accommodating various guest molecules inside its pores. MOFs possess robust physical attributes and exhibit resilience, offering the potential to assimilate many guest species [67].
MOFs possess the advantageous characteristic of being amenable to facile customization, synthesis, and design. When doing a comparative analysis between MOFs and other porous materials, such as activated carbon and zeolites, it becomes evident that MOFs provide a distinct advantage in readily optimizing the structure of pores, surface groups, and other associated features. Consequently, porous materials find use in many applications. MOFs may be classified into two distinct categories: stiff and namely flexible [68].
Flexible materials possess a dynamic and pliable framework that exhibits rapid responsiveness to many environmental stimuli, including changes in pressure, temperature fluctuations, and guest molecules. The exceptional responsiveness of MOFs to external stimuli enables them to exhibit distinctive characteristics, including pressure and temperature-dependent molecular sieving. As a result, MOFs surpass conventional adsorbents like zeolites and activated carbon in terms of performance [68].
In contrast, rigid MOFs have a robust and durable porous structure characterized by a stable and enduring porosity similar to zeolites. In recent years, rigid MOFs have been extensively used for the specific goal of selectively adsorbing gases. The selective adsorption process seen in rigid MOFs has a striking resemblance to that observed in zeolites. Consequently, the selective adsorption process may be used by leveraging the molecular sieving effect. The phenomenon of selective adsorption in rigid MOFs is influenced by three primary elements: separation, adsorption based on shape and size, and adsorption interactions. Furthermore, it is essential to consider the combined contribution of these aspects [69].
Numerous MOFs selectively adsorb selectively adsorb various gases, primarily relying on the molecular sieving action [70]. Table 1 presents the kinetic diameter of several gas species. This implies that only molecules with a kinetic diameter compatible with the dimensions of the MOF pores can traverse them [71].
MOFs exhibit remarkable porosity, which can be attained by self-assembly, including numerous metal ions and organic linkers, even under very gentle reaction conditions. Within the domain of porous materials, it is noteworthy that MOFs have a remarkable capacity for the adsorption of CO2, methane storage, and hydrogen adsorption by physical adsorption. These abilities transcend those of typical porous materials [73,74].
As a result, MOFs are widely used in carbon capture. Table 3 comprehensively analyzes different carbon adsorbents, delineating each variant's merits and drawbacks [75]. It is worth noting that MOFs provide the most significant working capacity among the carbon adsorbents that have been studied, as presented in Table 2.
Table 1. Kinetic diameter of some types of gas [72]
Table 2. Strengths and limitations of various CO2 adsorbents [75]
CO2 adsorption using metal-organic frameworks
Choosing a porous material for CO2 adsorption typically involves consideration of two primary factors: the selectivity of the adsorbent and the capacity of the material to adsorb CO2. Accordingly, it is anticipated that an optimal MOF will possess elevated adsorption capacity for CO2 gas compared to other gases, such as N2 and CH4, alongside a high overall adsorption capacity [76]. Table 3 provides the adsorption capacity, temperature, and pressure of the adsorption process using different types of MOF for CO2 capture. It is seen that with temperature elevation, the CO2 adsorption capacity diminishes [77]. The investigation has shown a decrease in adsorption capability as the temperature increases. In this context, the exponential function is the most suitable mathematical model to depict the correlation between temperature and the saturation absorption capacity. To achieve a significant increase in CO2 adsorption capacity, it is essential to ensure that the adsorption temperature is maintained at a suitably low level [78-89].
Table 3. Capacity, temperature, and pressure of CO2 adsorption using several metal-organic frameworks
CO2 adsorption in the biomass gasification process
The absorption and separation process has recently been considered an essential technology for reducing CO2 emissions. In other words, removing other acidic gases such as H2S, CO2, and COS from gas flows plays a prominent role in petrochemical, natural gas treatment, fossil fuel power plants, oil refineries, and the cement and steel industry [90]. There are various practical processes, such as chemical absorption, physical absorption, and physical separation. The standard techniques for CO2 removal are based on the absorption processes [91].
The syngas produced by gasification consist of CH4, H2, CO2, and CO, accompanied by tar. Notably, the presence of CO2 and CO in the syngas is considered undesirable. CO2 in the syngas results in a diminished thermal value of the syngas, while the tar contributes to the formation of sediments in the downstream equipment. Thus, these two components should be separated from the produced gas as fuel cell fuel [92].
Integration of the biomass gasification and CO2 adsorption
The process of integrating biomass with CO2 adsorption entails exothermic reactions. Therefore, it is imperative to conduct an energy analysis to facilitate the design of an efficient process and enable a comprehensive evaluation of its performance concerning other processes. In their study, Parviz et al. [93] conducted a comparative analysis of the performance of conventional gasification and rice husk gasification regarding energy and environmental considerations, explicitly focusing on the impact of increased CO2 levels. It was discovered that the exergy of the syngas exhibited an increase in correlation with the ratio of CO2 to biomass.
Previously, most research endeavors have focused on examining the integrated biomass gasification process in conjunction with CO2 adsorption, with air as the primary gasifying agent. These investigations have consistently yielded findings indicating a notably low hydrogen output. Most research has traditionally centered its attention on investigating the impact of operational factors on process performance, namely in terms of product distribution and energy efficiency. However, little needs to be conducted on the detailed examination of the combined process of integrated vapor biomass gasification and CO2 adsorption, whereby the captured CO2 is used to produce a gas rich in hydrogen.
A study investigated using wood residues as the primary feedstock for a comprehensive biomass gasification process, which included CO2 absorption [90]. This method had three main components: a regenerator, a carbonator, and a gasifier. To achieve complete absorption of the produced CO2, it is recommended to maintain a carbonate temperature of 500°C and a CaO/C ratio of more than 3.17. The CO and CO2 concentrations increased with the elevation of carbon temperature, while the hydrogen concentration dropped; when subjected to elevated temperatures, the carbonation reaction has less environmental effect since the resulting CH4 is wholly consumed [94].
The integrated process has also been investigated in the context of CO2 recirculation, whereby the captured CO2 is employed as a gasifying agent. The findings of this research indicate that the gas produced by the PEMFC specifications can be generated for any CO2/C ratios, provided that the carbonation temperature remains below 500 ˚C [95]. In the context of the biomass gasification process, it can be seen that the concentration of H2 is consistently higher when using adsorption and recirculation of CO2, as compared to the standard biomass gasification process. This trend is particularly evident when the carbonation temperature remains below 750 ˚C [95].
Since the integrated biomass gasification process and CO2 adsorption can enhance hydrogen-rich gas production, this process has gained much attention. Acharia et al. dealt with hydrogen production through biomass gasification with in situ CO2 adsorption via CaO through pilot execution using a fluidized bed vapor gasifier. In that study, sawdust was considered as the feed. The synthase gas was obtained with a high hydrogen concentration of 71% and a low CO2 concentration of almost zero [17].
In addition, the effect of adding CaO was studied on tar formation and hydrogen production in a bubbled fluidized bed. In the CaO presence, the hydrogen concentration increased by 20%, while that of tar dropped by 67% [96].
In another study, Mahishi and Gosoami studied a new technique involving integrating adsorption and gasification reactions to enhance the yield of the generated hydrogen in the conventional biomass vapor gasification process. In this technique, gasification of carbonated fuel was done as biomass in the presence of vapor and CO2 adsorbent [97].
Conclusion
Given the consequences of global warming and the energy crisis, attempts to reduce greenhouse gas emissions, including CO2, and increasing the use of renewable fuels and generating clean fuels are crucial.
The development of biomass gasification technology for improving the energy structure, reducing greenhouse gas emissions, and developing a low-carbon green economy are significant. It would also reduce dependence on fossil fuels.
One of the most essential uses of biomass gasification is the generation of high-purity hydrogen through the absorption and separation of CO2. Although there are novel technologies for CO2 removal, the adsorption process is the most popular CO2 separation method from flue gas or natural gas.
This review discussed the integration of biomass gasification and the adsorption process, using metal-organic frameworks as CO2 adsorbents, as a new generation of adsorbents. Compared with traditional porous materials such as activated carbon and zeolite, MOFs, due to their adjustable structures, suitable porosity, and chemical bonds, offer more significant advantages in CO2 adsorption. Fortunately, MOF features are constantly being improved and expanded over time, allowing them to be used as a suitable adsorbent for CO2 capture. Through continuous studies, the challenges related to the adsorption process could be overcome, and the CO2 adsorption process could be integrated with the current industrial systems.
Conflict of interest
No potential conflict of interest was reported by the authors.
Orcid
Mohammadsaleh Hoseinzadeh : 0000-0001-6808-6937
Fahimeh Hooriabad Saboor : 0000-0001-7729-0673
- Cheng, Z. Thow, C.-H. Wang, Powder Technol., 2016, 296, 87-101. [CrossRef], [Google Scholar], [Publisher]
- M. Rudin, Z. Muis, H. Hashim, W.S. Ho, Chem. Eng. Trans., 2017, 56, 649-654. [CrossRef], [Google Scholar], [Publisher]
- E. Resasco, B. Wang, D. Sabatini, Nat. Catal., 2018, 1, 731-735. [CrossRef], [Google Scholar], [Publisher]
- R.I. Sardara, F. Hasan, M.J. Alama, I.H. Nadima, M. Mahmuda, Optim., 2023, 3, 108-122. [CrossRef], [Google Scholar], [Publisher]
- Zhang, J. Li, L. Guo, Z. Chen, C. Li, Appl. Catal., B, 2018, 237, 660-664. [CrossRef], [Google Scholar], [Publisher]
- A. Situmorang, Z. Zhao, A. Yoshida, A. Abudula, G. Guan, Renew. Sust. Energ. Rev., 2020, 117, 109486. [CrossRef], [Google Scholar], [Publisher]
- Bello, C.M. Agu, D.A. Ajiya, A.A. Mahmoud, L. Udopia, N.M. Lawal, A.A. Abdullahi, M. Muhammad, J. Chem. Rev., 2022, 4, 272-287. [CrossRef], [Google Scholar], [Publisher]
- Kargbo, J.S. Harris, A.N. Phan, Renew. Sust. Energ. Rev., 2021, 135, 110168. [CrossRef], [Google Scholar], [Publisher]
- Li, J. Xu, H. Xie, Y. Wang, Energy Convers. Manag., 2018, 172, 554-566. [CrossRef], [Google Scholar], [Publisher]
- L. Yiin, A.T. Quitain, S. Yusup, Y. Uemura, M. Sasaki, T. Kida, Bioresour. Technol., 2018, 261, 361-369. [CrossRef], [Google Scholar], [Publisher]
- Remón, P. Arcelus-Arrillaga, L. García, J. Arauzo, Appl. Energy, 2018, 228, 2275-2287. [CrossRef], [Google Scholar], [Publisher]
- Ahmad, K. Kainat, U. Farooq, J. Chem. Rev., 2022, 4, 374-422. [CrossRef], [Google Scholar], [Publisher]
- Ahrenfeldt, T.P. Thomsen, U. Henriksen, L.R. Clausen, Appl. Therm. Eng., 2013, 50, 1407-1417. [CrossRef], [Google Scholar], [Publisher]
- Kalinci, A. Hepbasli, I. Dincer, Int. J. Hydrogen Energy., 2009, 34, 8799-8817. [CrossRef], [Google Scholar], [Publisher]
- Cao, K. Iris, X. Xiong, D.C. Tsang, S. Zhang, J.H. Clark, C. Hu, Y.H. Ng, J. Shang, Y.S. Ok, Environ. Res., 2020, 186, 109547. [CrossRef], [Google Scholar], [Publisher]
- Luis, Desalination, 2016, 380, 93-99. [CrossRef], [Google Scholar], [Publisher]
- R. Li, Y. Ma, M.C. McCarthy, J. Sculley, J. Yu, H.K. Jeong, P.B. Balbuena, H.-C. Zhou, Coord. Chem. Rev., 2011, 255, 1791-1823. [CrossRef], [Google Scholar], [Publisher]
- D. Farahani, V.S. Fard, J. Appl. Organomet. Chem., 2022, 2, 180-194. [CrossRef], [Google Scholar], [Publisher]
- Acharya, A. Dutta, P. Basu, Energy & Fuels, 2009, 23, 5077-5083. [CrossRef], [Google Scholar], [Publisher]
- Balat, E. Kırtay, Int. J. Hydrog. Energy, 2010, 35, 7416-7426. [CrossRef], [Google Scholar], [Publisher]
- Wang, H. Jin, H. Feng, W. Wei, C. Cao, W. Cao, Int. J. Hydrog. Energy, 2020, 45, 28051-28061. [CrossRef], [Google Scholar], [Publisher]
- Faraji, M. Saidi, Int. J. Hydrog. Energy, 2021, 46, 18844-18856. [CrossRef], [Google Scholar], [Publisher]
- S. Siwal, Q. Zhang, C. Sun, S. Thakur, V.K. Gupta, V.K. Thakur, Bioresour. Technol., 2020, 297, 122481. [CrossRef], [Google Scholar], [Publisher]
- Inayat, M.M. Ahmad, S. Yusup, M.I.A. Mutalib, Energies, 2010, 3, 1472-1484. [CrossRef], [Google Scholar], [Publisher]
- Dutta, V. Pandey, A. Das, S. Sen, D. Baruah, Energy Procedia, 2014, 54, 21-34. [CrossRef], [Google Scholar], [Publisher]
- Beheshti, H. Ghassemi, R. Shahsavan-Markadeh, Energy Convers. Manag., 2015, 94, 345-352. [CrossRef], [Google Scholar], [Publisher]
- Fremaux, S.-M. Beheshti, H. Ghassemi, R. Shahsavan-Markadeh, Energy Convers. Manag., 2015, 91, 427-432. [CrossRef], [Google Scholar], [Publisher]
- Asadullah, Renew. Sust. Energ. Rev., 2014, 29, 201-215. [CrossRef], [Google Scholar], [Publisher]
- P. Shukla, echnologies for sustainable rural development: having potential of socio-economic upliftment, Allied Publishers, 2014. [Google Scholar], [Publisher]
- Borthakur, D. Mahanta, Int. J. Sci. Res., 2013, 2, 276-278. [Google Scholar]
- Yuan, M.D. Boyette, D. Wang, A. Kumar, Int. J. Agric. Biol. Eng., 2014, 7, 91-97. [Google Scholar], [Publisher]
- Ramzan, M. Athar, S. Begum, S.W. Ahmad, S. Naveed, Pol. J. Chem. Technol., 2015, 17, 66-78. [CrossRef], [Google Scholar], [Publisher]
- A. Olajire, Energy, 2010, 35, 2610-2628. [CrossRef], [Google Scholar], [Publisher]
- Anwar, A. Fayyaz, N. Sohail, M. Khokhar, M. Baqar, W. Khan, K. Rasool, M. Rehan, A. Nizami, J. Environ. Manage., 2018, 226, 131-144. [CrossRef], [Google Scholar], [Publisher]
- Aghaie, N. Rezaei, S. Zendehboudi, Renew. Sust. Energ. Rev., 2018, 96, 502-525. [CrossRef], [Google Scholar], [Publisher]
- Ben-Mansour, M. Habib, O. Bamidele, M. Basha, N. Qasem, A. Peedikakkal, T. Laoui, M. Ali, Appl. Energy, 2016, 161, 225-255. [CrossRef], [Google Scholar], [Publisher]
- Y. Leung, G. Caramanna, M.M. Maroto-Valer, Renew. Sust. Energ. Rev., 2014, 39, 426-443. [CrossRef], [Google Scholar], [Publisher]
- M. Cuéllar-Franca, A. Azapagic, J. CO2 Util., 2015, 9, 82-102. [CrossRef], [Google Scholar], [Publisher]
- Y. Hong, CCST, 2022, 3, 100044. [CrossRef], [Google Scholar], [Publisher]
- Zhang, Z. Song, R. Gani, T. Zhou, Ind. Eng. Chem. Res., 2020, 59, 2005-2012. [CrossRef], [Google Scholar], [Publisher]
- Samanta, A. Zhao, G.K. Shimizu, P. Sarkar, R. Gupta, Ind. Eng. Chem. Res., 2012, 51, 1438-1463. [CrossRef], [Google Scholar], [Publisher]
- El Hadri, D.V. Quang, E.L. Goetheer, M.R.A. Zahra, Appl. Energy, 2017, 185, 1433-1449. [CrossRef], [Google Scholar], [Publisher]
- Ghiat, A. AlNouss, G. McKay, T. Al-Ansari, Chem. Eng., 2020, 135, 106758. [CrossRef], [Google Scholar], [Publisher]
- Khan, S. Yusup, M. Aslam, A. Inayat, M. Shahbaz, S.R. Naqvi, R. Farooq, I. Watson, J. Clean. Prod., 2019, 236, 117636. [CrossRef], [Google Scholar], [Publisher]
- Shahbaz, S. Yusup, A. Inayat, M. Ammar, D.O. Patrick, A. Pratama, S.R. Naqvi, Energy & Fuels, 2017, 31, 12350-12357. [CrossRef], [Google Scholar], [Publisher]
- M. White, B.R. Strazisar, E.J. Granite, J.S. Hoffman, H.W. Pennline, J. Air Waste Manag. Assoc., 2003, 53, 645-715. [CrossRef], [Google Scholar], [Publisher]
- Song, Q. Liu, S. Deng, H. Li, Y. Kitamura, Renew. Sust. Energ. Rev., 2019, 101, 265-278. [CrossRef], [Google Scholar], [Publisher]
- Babu, P. Linga, R. Kumar, P. Englezos, Energy, 2015, 85, 261-279. [CrossRef], [Google Scholar], [Publisher]
- Bui, M. Fajardy, N. Mac Dowell, Appl. Energy, 2017, 195, 289-302. [CrossRef], [Google Scholar], [Publisher]
- Mohamedali, D. Nath, H. Ibrahim, A. Henni, Review of recent developments in CO2 capture using solid materials: metal organic frameworks In Greenhouse Gases, 2016, pp. 115-154. [Google Scholar], [Publisher]
- Brenner, H., Adsorption Calculations and Modelling, Elsevier Science: 2013. [CrossRef], [Google Scholar]
- Farrusseng, Metal-organic frameworks: applications from catalysis to gas storage, John Wiley & Sons, 2011. [Google Scholar]
- Steele, G. Zgrablich, W. Rudzinski, Equilibria and dynamics of gas adsorption on heterogeneous solid surfaces, Elsevier, 1996. [Google Scholar]
- W. Chester, E.G. Derouane, Zeolite characterization and catalysis, Springer, 2009. [CrossRef], [Google Scholar], [Publisher]
- Primo, H. Garcia, Chem. Soc. Rev., 2014, 43, 7548-7561. [CrossRef], [Google Scholar], [Publisher]
- Cavenati, C.A. Grande, A.E. Rodrigues, Energy & fuels, 2006, 20, 2648-2659. [CrossRef], [Google Scholar], [Publisher]
- Merel, M. Clausse, F. Meunier, Ind. Eng. Chem. Res., 2008, 47, 209-215. [CrossRef], [Google Scholar], [Publisher]
- Remy, S.A. Peter, L. Van Tendeloo, S. Van der Perre, Y. Lorgouilloux, C.E. Kirschhock, G.V. Baron, J.F. Denayer, Langmuir, 2013, 29, 4998-5012. [CrossRef], [Google Scholar], [Publisher]
- Su, C. Lu, Energy Environ. Sci., 2012, 5, 9021-9027. [CrossRef], [Google Scholar], [Publisher]
- H. Hong, M.S. Jang, S.J. Cho, W.S. Ahn, Chem. Comm., 2014, 50, 4927-4930. [CrossRef], [Google Scholar], [Publisher]
- J. Bandosz, Activated carbon surfaces in environmental remediation, Elsevier, 2006. [Google Scholar]
- S. Novoselov, A.K. Geim, S.V. Morozov, D.e. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, science, 2004, 306, 666-669. [CrossRef], [Google Scholar], [Publisher]
- Gadipelli, Z.X. Guo, Prog. Mater. Sci., 2015, 69, 1-60. [CrossRef], [Google Scholar], [Publisher]
- Li, X. Jiang, J. Zhao, S. Zhang, Nano energy, 2015, 16, 488-515. [CrossRef], [Google Scholar], [Publisher]
- L.M. Reddy, S. Ramaprabhu, Int. J. Hydrogen Energy, 2008, 33, 1028-1034. [CrossRef], [Google Scholar], [Publisher]
- Hu, L. Wang, S. Zhang, Y. Wang, F. Jin, X. Fu, H. Li, J. Environ. Sci., 2014, 26, 1709-1716. [CrossRef], [Google Scholar], [Publisher]
- Younas, M. Rezakazemi, M. Daud, M.B. Wazir, S. Ahmad, N. Ullah, S. Ramakrishna, PECS, 2020, 80, 100849. [CrossRef], [Google Scholar], [Publisher]
- S. Qin, S. Yuan, A. Alsalme, H.C. Zhou, ACS Appl. Mater. Interfaces, 2017, 9, 33408-33412. [CrossRef], [Google Scholar], [Publisher]
- R. Li, R.J. Kuppler, H.C. Zhou, Chem. Soc. Rev., 2009, 38, 1477-1504. [CrossRef], [Google Scholar], [Publisher]
- Zhuang, D. Yuan, D. Liu, C. Zhong, J.R. Li, H.C. Zhou, Chem. Mater., 2012, 24, 18-25. [CrossRef], [Google Scholar], [Publisher]
- E. Kentish, C.A. Scholes, G.W. Stevens, Recent Patents on Chemical Engineering, 2008, 1, 52-66. [CrossRef], [Google Scholar], [Publisher]
- Abdirakhimov, M.H. Al-Rashed, J. Wójcik, Energies, 2022, 15, 5391. [CrossRef], [Google Scholar], [Publisher]
- A. Qasem, R. Ben-Mansour, M.A. Habib, Appl. Energy, 2018, 210, 317-326. [CrossRef], [Google Scholar], [Publisher]
- Joharian, A. Morsali, J. Solid State Chem., 2019, 270, 135-146. [CrossRef], [Google Scholar], [Publisher]
- Mukherjee, A. Kumar, M.J. Zaworotko, Elsevier, 2019, 5-61. [CrossRef], [Google Scholar], [Publisher]
- Zou, G. Zhu, Microporous Materials for Separation Membranes, Weinheim: Germany, Wiley-VCH Verlag GmbH & Co. KGaA, 2019, pp. 323-359. [Google Scholar]
- Li, X. Ren, X. Feng, X. Li, C. Hu, B. Wang, Chem. Comm., 2014, 50, 6894-6897. [CrossRef], [Google Scholar], [Publisher]
- Hayashi, A.P. Côté, H. Furukawa, M. O’Keeffe, O.M. Yaghi, Nat. Mater., 2007, 6, 501-506. [CrossRef], [Google Scholar], [Publisher]
- Yu, P.B. Balbuena, J. Phys. Chem. C, 2013, 117, 3383-3388. [CrossRef], [Google Scholar], [Publisher]
- Park, J.-R. Li, Y.-P. Chen, J. Yu, A.A. Yakovenko, Z.U. Wang, L.-B. Sun, P.B. Balbuena, H.-C. Zhou, Chem. Comm., 2012, 48, 9995-9997. [CrossRef], [Google Scholar], [Publisher]
- Ma, X.-S. Wang, E.S. Manis, C.D. Collier, H.-C. Zhou, Inorg. Chem., 2007, 46, 3432-3434. [CrossRef], [Google Scholar], [Publisher]
- H. Hong, M.P. Suh, Chem. Comm., 2012, 48, 9168-9170. [CrossRef], [Google Scholar], [Publisher]
- Bourrelly, P.L. Llewellyn, C. Serre, F. Millange, T. Loiseau, G. Férey, J. Am. Chem. Soc., 2005, 127, 13519-13521. [CrossRef], [Google Scholar], [Publisher]
- L. Llewellyn, S. Bourrelly, C. Serre, Y. Filinchuk, G. Férey, Angew. Chem., 2006, 118, 7915-7918. [CrossRef], [Google Scholar], [Publisher]
- Bataille, S. Bracco, A. Comotti, F. Costantino, A. Guerri, A. Ienco, F. Marmottini, Cryst. Eng. Comm., 2012, 14, 7170-7173. [CrossRef], [Google Scholar], [Publisher]
- Yan, Y. Lin, P. Wu, L. Zhao, L. Cao, L. Peng, C. Kong, L. Chen, Chem. Plus. Chem., 2013, 78, 86-91. [CrossRef], [Google Scholar], [Publisher]
- R. Wade, M. Dincă, J. Chem. Soc., Dalton Trans., 2012, 41, 7931-7938. [CrossRef], [Google Scholar], [Publisher]
- Y. Masoomi, K.C. Stylianou, A. Morsali, P. Retailleau, D. Maspoch, Cryst. Growth Des., 2014, 14, 2092-2096. [CrossRef], [Google Scholar], [Publisher]
- Saidi, A. Omri, Prog. Nucl. Energy, 2020, 126, 103425. [CrossRef], [Google Scholar], [Publisher]
- M. Saleh, A.I. Hassan, Fire, 2023, 6, 128. [CrossRef], [Google Scholar], [Publisher]
- Chen, L. Jin, X. Chen, Procedia Eng., 2011, 26, 126-131. [CrossRef], [Google Scholar], [Publisher]
- H. Florin, A.T. Harris, Chem. Eng. Sci., 2008, 63, 287-316. [CrossRef], [Google Scholar], [Publisher]
- Parvez, I.M. Mujtaba, T. Wu, Energy, 2016, 94, 579-588. [CrossRef], [Google Scholar], [Publisher]
- Xiong, Y. Yang, F. Xu, Y. Pan, J. Zhang, K. Hong, G. Lorenzini, S. Wang, ACS Sustain. Chem. Eng., 2017, 5, 2783-2798. [CrossRef], [Google Scholar], [Publisher]
- Basu, Biomass gasification, pyrolysis and torrefaction: practical design and theory, Academic press, 2018. [Google Scholar]
- Detchusananard, K. Im-orb, P. Ponpesh, A. Arpornwichanop, Energy Convers. Manag., 2018, 171, 1560-1572. [CrossRef], [Google Scholar], [Publisher]
- M.R. Mahishi, D. Goswami, Int. J. Hydrogen Energy., 2007, 32, 3831-3840. [CrossRef], [Google Scholar], [Publisher]